Insights on the disruption of glucose metabolism and hepatic insulin resistance induced by hepatitis C virus
Abstract
Hepatitis C virus (HCV) is still considered as a major public health problem because in 2015 around 71 million people were chronically infected worldwide. It is important to note that chronic HCV infection is a systemic disease that is associated with diverse extrahepatic disorders including insulin resistance and type 2 diabetes mellitus. The discovery of new direct-acting antiviral agents (DAAs) has become a huge advance in the treatment of HCV infection. The complex interplay between HCV and glucose metabolic pathways remains to be fully elucidated, but it is becoming clearer that elimination of chronic HCV infection halts the progression of liver disease, but more evidence is still needed to better understand how successful antiviral treatment influences insulin resistance and other abnormalities of glucose metabolism linked to HCV infection. This review provides a comprehensive overview of the glucose metabolism disturbances related to chronic HCV infection, highlighting the new insights into the molecular basis of insulin resistance induced by HCV and the mechanisms underlying the reversion of this metabolic disorder by DAAs.
Keywords
INTRODUCTION
Hepatitis C virus (HCV) infection is a systemic disease which is associated with diverse extrahepatic disorders including atherosclerosis, lipid metabolic disturbances, lymphoproliferative diseases, and glucose metabolic alterations leading to insulin resistance and type 2 diabetes mellitus (T2DM). A great body of clinical and experimental evidence indicating a close relationship between insulin resistance and chronic HCV infection exists, and it is well known that insulin resistance-associated fatty liver disease contributes to the progression of HCV-induced liver disease by enhancing the virulence of HCV and inducing cellular and molecular mechanisms involved in hepatic fibrogenesis. The complex interplay between HCV and glucose metabolism remains to be fully elucidated, but it is becoming clearer that elimination of chronic HCV infection after direct-acting antiviral (DAA)-based therapy halts the progression of liver disease. In this review, besides a comprehensive overview of the glucose metabolism disturbances related to chronic HCV infection, we want to highlight the new insights into the molecular basis of insulin resistance induced by HCV and the mechanisms underlying the reversion of this metabolic disorder by DAAs.
EVOLVING EPIDEMIOLOGY OF HEPATITIS C INFECTION: FROM THE ERA OF INTERFERON ALPHA TO DIRECT-ACTING ANTIVIRALS
According to the most recent and accurate study on the prevalence of HCV chronic infection, which was promoted by the World Health Organization (WHO) Hepatitis Report and published in 2018, HCV infection was estimated to affect 71 million individuals worldwide in 2015, more than 1% of the world’s population[1]. The countries with the highest prevalence are China, Pakistan, India, Egypt, and Russia. In fact, these countries alone sum up more than 45% of infected patients. The European continent has an estimated 14 million infected individuals. The reported incidence of HCV infection in 2015 was 1.75 million new cases, and the number of deaths attributed to HCV alone is 400,000 per year[1].
Hepatitis C virus is a single-stranded RNA virus that belongs to the Hepacivirus genus and the Flaviviridae family. Currently, there are eight genotypes of HCV[2]. The prevalence of the different genotypes varies from one geographical region to another: Genotype 1 is the most prevalent (46%) and predominates in North America, Europe, and Australia, followed by Genotype 3 (30%) mainly distributed in South Asia. Genotypes 2, 4, and 6 encompass approximately 23% of cases. Finally, Genotypes 5, 7, and 8 comprise less than 1%[2,3]. The first treatment for HCV infection was approved in 1991. Interferon alpha (IFN) 2b, administered at a dose of 3 million units three times per week for 6-12 months, provided a sustained viral response (SVR) of around 15%-20% with relevant and sometimes limiting side effects. In the late 1990s, the addition of daily oral ribavirin rose the SVR to 38%[4], and, in the early 2000s, the introduction of weekly pegylated (PEG)-IFN 2a or 2b increased the SVR rate to over 50%[5]. After many years without relevant changes in HCV therapy, in 2011, DAAs were approved for the treatment of chronic HCV infection. The first generation of DAAs included the NS3/NS4A protease inhibitors telaprevir and boceprevir, which together with PEG-IFN and ribavirin, achieved an SVR of 75% in patients with Genotype 1 HCV infection[6]. Up to this point, the addition of more medications to the therapy to increase SVR was followed by a progressive increase of significant side effects. The second generation appeared in 2014: protease inhibitors or NS5A or NS5B polymerase inhibitors were the first IFN-free regimens, entailing a turning point in HCV therapy[7]. The most remarkable breakthrough was the introduction of sofosbuvir (SOF), an NS5B polymerase inhibitor[8,9]. This DAA used in combination with PEG-INF and ribavirin showed a high rate of SVR up to 90% in non-treated patients with Genotype 1 or 4 after 12 weeks of treatment with no additional adverse effects such as fatigue, headache, nausea, and anemia compared to PEG-IFN and ribavirin regimen (NEUTRINO Trial[10]). Moreover, 12 weeks of SOF and ribavirin treatment was as effective as PEG-IFN and ribavirin treatment in non-treated patients with Genotype 2 or 3 (SVR of 67%)[10]. The results from PHOTON-1 study reveal that treatment with SOF and ribavirin was effective in improving SRV rates in HIV/HCV co-infected patients[11].
At this point, there were several specific regimens effective for each genotype. More recently, a third generation of pan-genotypic DAAs, effective for all HCV genotypes, has appeared[12]. SVR is achieved with modern DAAs in 90%-98% of infected patients. However, around 3%-4% of patients develop resistant variants. These variants are developed in most cases in previously treated individuals, but some cases might also occur in treatment-naïve patients.
DISRUPTION OF GLUCOSE METABOLISM ASSOCIATED WITH CHRONIC HEPATITIS C VIRUS INFECTION
Even though its main target is the liver, HCV infection is considered a systemic disease. Indeed, over 70% of HCV infected patients develop at least one extrahepatic manifestation. Many of these manifestations have been described, but the strength of the data proving a correlation with HCV varies. In this review, we focus on the metabolic manifestations associated with HCV, particularly on those affecting glucose metabolism.
Firstly, it has been described that cirrhotic patients with HCV were more likely to have T2DM than those with cirrhosis due to other causes[13,14]. Then, several clinical studies have shown a higher prevalence of T2DM in patients with HCV compared to healthy controls[15-18]. The odds ratio of T2DM among HCV-infected subjects is about 1.2-1.7 times than that of healthy subjects[18]. A systematic review and meta-analysis of the existing data done in 2018 showed that the pooled prevalence of T2DM among HCV-infected patients globally was 19.67% (95%CI: 17.25-22.09), while the global prevalence of T2DM (8.5%) among the general population was much lower. The prevalence rate of T2DM in HCV patients ranges from 15% to 28%, with the highest prevalence rate in Africa and Asia, while the lowest prevalence rate is in Europe[19]. Noteworthy, the prevalence of insulin resistance and T2DM is significantly higher in HCV-infected patients compared to other chronic liver diseases such as HBV infection, alcoholic liver disease, and primary biliary cirrhosis[20,21]. Moreover, several studies have demonstrated that HCV-associated hepatic insulin resistance can negatively affect the treatment response with IFN-based therapies[22-24].
It is important to note that a significant improvement was observed in the systemic insulin response in HCV patients after virus eradication, regardless of the treatment. A decrease in insulin resistance, as estimated by homeostasis model assessment (HOMA-IR), has been described and seems to be associated with viral clearance after IFN-based regimens [Table 1]. Overall, several studies have demonstrated that HCV patients who achieved SVR showed a reduction of HOMA-IR index during and after treatment compared to non-responder patients regardless of HCV genotype[24-26]. Similar results were reported using other homeostasis model assessments such as the HOMA2-IR[27]. However, Thompson et al.[28] only observed a decrease of HOMA-IR in sustained responders with HCV Genotype 1 but not in those with Genotype 2 or 3. Other studies reported that HOMA-IR levels decreased only in insulin-resistant patients but were unrelated to treatment outcomes[29,30] or even remained unchanged[31]. One possible explanation for these conflicting results is the different population characteristics, viral factors, and/or the duration of the follow-up period. Notably, the incidence of T2DM and/or the development of glucose abnormalities such as impaired fasting glucose significantly decreased in those HCV patients who eradicated the virus after treatment with IFN-based regimens[32-34].
Principal clinical studies examining the impact of sustained virological response by IFN-based antiviral therapy on glucose metabolism
| Study | Study population | Demographics Age/Sex/Race | Study design | Primary endpoints | Clinical outcomes |
| Romero-Gómez et al.[24] 2005 | 50 HCV non-diabetic patients | 43 ± 10 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values in SVR patients |
| Simó et al.[34] 2006 | 96 HCV non-diabetic patients | 42 ± 10 years/male > female/N.A. | Prospective observational longitudinal study | Incidence of T2DM | T2DM incidence was significantly reduced (P < 0.05) in SVR patients |
| Kawaguchi et al.[25] 2007 | 89 HCV non-diabetic patients | 60 ± 9 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values in SVR patients |
| Lo Iacono et al.[30] 2007 | 48 HCV non-diabetic patients | 50 ± 12 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR | HOMA-IR values significantly decreased (P < 0.05) but unrelated to treatment outcomes |
| Romero-Gómez et al.[33] 2008 | 734 HCV non-diabetic patients | 53 ± 10 years/male > female/N.A. | Prospective observational longitudinal study | Incidence of T2DM/IFG | T2DM/IFG incidence was significantly reduced (p < 0.05) in SVR patients |
| Arase et al.[32] 2009 | 2842 non-diabetic HCV patients | 52 ± 9 years/male > female/N.A. | Prospective observational longitudinal study | Incidence of T2DM | T2DM incidence was significantly reduced (P < 0.05) in SVR patients |
| Delgado-Borrego et al.[27] 2010 | 96 HCV non-diabetic patients | 49 ± 11 years/male > female/White predominant | Prospective observational longitudinal study | HOMA2-IR | Significant reduction (P < 0.05) of HOMA2-IR values in SVR patients |
| Thompson et al.[28] 2012 | 1038 non-diabetic HCV patients | 46 ± 9 years/male > female/White predominant | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values only in SVR genotype 1 patients |
| Aghemo et al.[31] 2012 | 309 non-diabetic HCV patients | 52 ± 12 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR | No significant variations of HOMA-IR values (P > 0.05) |
| Khattab et al.[26] 2012 | 107 non-diabetic HCV patients | 41 ± 6 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values in SVR patients |
| Chien et al.[29] 2015 | 78 HCV non-diabetic patients | 54 ± 12 years/female > male/N.A. | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values only in patients with pretreatment insulin resistance, but unrelated to treatment outcomes |
On the other hand, several reports have demonstrated that antiviral therapy with DAAs, including SOF, improved systemic insulin resistance and glucose homeostasis in patients who cleared HCV [Table 2]. DAA-based eradication of HCV is associated with improved glycemic control in patients with established T2DM, as demonstrated by the reduction of fasting glucose and HbA1c levels[35-38]. In this line, we recently published that SOF-based treatments effectively improve the insulin-resistant status of patients with chronic HCV infection, regardless of HCV genotype or degree of liver fibrosis. Interestingly, the significant reduction of post-treatment HOMA-IR index observed was maintained one year later[39].
Principal clinical studies examining the impact of HCV clearance by DAAs on glucose metabolism
| Study | Study population | Demographics Age/Sex/Race | Study design | Primary endpoints | Clinical outcomes |
| Hum et al.[37] 2017 | 2435 HCV diabetic patients | 62 ± 5 years/male > female/Black predominant | Prospective observational longitudinal study | HbA1c | Significant reduction (P < 0.05) of HbA1c values in SVR patients |
| Ciancio et al.[36] 2018 | 122 HCV diabetic patients | 61 ± 11 years/male > female/N.A. | Prospective observational longitudinal study | Fasting glucose and HbA1c | Significant reduction (P < 0.05) of fasting glucose and HbA1c values in SVR patients |
| Tada et al.[41] 2018 | 198 HCV non-diabetic patients | 72 ± 5 years/female > male/N.A. | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values in SVR patients |
| Carvalho et al.[43] 2018 | 105 HCV non-diabetic patients | 53 ± 9 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR and fasting glucose | Significant reduction (P < 0.05) of HOMA-IR, but not of fasting glucose values in SVR patients |
| Boraie et al.[35] 2019 | 120 HCV diabetic patients | 52 ± 8 years/N.A./N.A. | Prospective observational longitudinal study | Fasting glucose, HbA1c and HOMA-IR | Significant reduction (P < 0.05) of fasting glucose, HbA1c, and HOMA-IR values in SVR patients |
| Zied et al.[38] 2020 | 100 HCV diabetic patients | 53 ± 10 years /N.A./N.A. | Prospective observational longitudinal study | Fasting glucose and HbA1c | Significant reduction (P < 0.05) of fasting glucose and HbA1c values in SVR patients |
| Ribaldone et al.[45] 2020 | 66,769 HCV non-diabetic patients | N.A./N.A./N.A. | Systematic review and meta-analysis | Incidence of T2DM | T2DM incidence was significantly reduced (P < 0.05) |
| Adinolfi et al.[40] 2020 | 2564 HCV non-diabetic patients | 68 ± 9 years/female > male/N.A. | Prospective observational longitudinal study | Incidence of T2DM | T2DM incidence was significantly reduced (P < 0.05) |
| Adinolfi et al.[40] 2020 | 384 HCV non-diabetic patients | 66 ± 8 years/female > male/N.A. | Prospective observational longitudinal study | HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR values in SVR patients |
| Russo et al.[44] 2020 | 138 HCV non-diabetic patients | 58 ± 10 years/male > female/N.A. | Prospective observational longitudinal study | Fasting glucose, HbA1c and HOMA-IR | Significant reduction (P < 0.05) of HOMA-IR, but not of fasting glucose and HbA1c values in SVR patients |
| Nevola et al.[42] 2020 | 269 HCV non-diabetic patients | 68 ± 7 years/female > male/N.A. | Prospective observational longitudinal study | HOMA-IR and fasting glucose | Significant reduction (P < 0.05) of fasting glucose and HOMA-IR values in SVR patients |
| Sacco et al.[46] 2021 | 68,096 HCV non-diabetic patients | N.A./N.A./N.A. | Systematic review and meta-analysis | Incidence of T2DM | T2DM incidence was significantly reduced (P < 0.05) |
| Rey et al.[39] 2021 | 42 HCV insulin-resistant patients | 54 ± 10 years/male > female/N.A. | Prospective observational longitudinal study | HOMA-IR and fasting glucose | Significant reduction (P < 0.05) of fasting glucose and HOMA-IR values in SVR patients |
Several studies have reported that clearance of HCV by DAAs resulted in a decrease in insulin resistance index, as assessed by HOMA-IR[40,41], and improved glucose abnormalities such as elevated levels of fasting glucose and HbA1c in non-diabetic patients[35,42]; conversely, some studies have observed only a reduction of HOMA-IR values in HCV patients after DDA therapy but without changes in other clinical outcomes related with glycemic control[43,44]. The latter studies had a shorter post-treatment follow-up period than the previous ones, which could explain the discrepancies observed because a longer observation time is required to properly evaluate the effects of HCV elimination on glucose homeostasis.
To highlight, two recent systematic reviews have revealed a significant reduction in the incidence of T2DM in non-diabetic HCV patients achieving SVR after DAA treatment, and the metabolic control significantly improved in diabetic HCV patients who eradicate the virus after DAAs therapy[45,46].
MOLECULAR MECHANISMS INVOLVED IN THE HEPATIC INSULIN RESISTANCE INDUCED BY CHRONIC HEPATITIS C VIRUS INFECTION
The increased prevalence of T2DM in HCV-infected patients may be due not only to the development of insulin resistance because of the disruption of insulin signaling but also to beta-cell failure and altered microRNAs, which are closely linked to the development of T2DM and other metabolic diseases[47].
MicroRNAs (miRNAs) play an important role in HCV replication, and host miRNA expression is altered during HCV infection. Differential expression of 108 miRNAs has been identified in hepatocytes after acute HCV infection. HCV increases the expression of miR-27 family members, which are involved in the regulation of lipid metabolism and contribute to the development of liver steatosis. HCV also increases circulating miR-122 content. The elevated levels of this miRNA have been closely related to the risk of developing metabolic syndrome and T2DM in the general population[47,48].
Among the peripheral tissues involved in the control of glucose homeostasis, the liver plays a major role because this organ has the ability to consume and produce glucose[49]. In this regard, besides the liver, both skeletal muscle and adipose tissue are the major sites of insulin resistance in HCV infections; however, in this review, we focus on the molecular mechanisms responsible for hepatic insulin resistance mediated by HCV.
The molecular mechanism underlying hepatic insulin resistance involves the impairment of the insulin receptor (IR) signaling network. The cascade begins when the IR is activated by autophosphorylation on several tyrosine residues leading to the recruitment and tyrosine phosphorylation of insulin receptor substrate (IRS) proteins such as IRS1 and IRS2[50].
It is well known that HCV interferes with the early steps of the insulin signaling cascade, particularly by reducing the expression of IRS proteins, IRS1 and IRS2. These docking proteins are key mediators of insulin and insulin-like growth factor 1 (IGF1) signaling, integrating essential signals from the IR and IGF1 receptor that regulate a variety of processes including metabolism, cellular growth, development, and survival[50], since there are two main pathways emerging from the activated IRS proteins: the phosphatidylinositol 3 kinase/AKT and mitogen-activated protein kinase pathways[51]. Inactivation of these proteins by different mechanisms, such as proteasome-mediated degradation, has been highly associated with insulin resistance[52]. In this regard, it is well established that HCV core protein induces serine phosphorylation of IRS1 blocking its tyrosine phosphorylation and targets IRS1 for proteosomal degradation. The activation of both c-jun kinase and mTOR/S6K axes has been found to be involved in IRS1 serine phosphorylation prompted by HCV[53]. Besides IRS1, degradation of IRS2 has been described in livers of patients with HCV infection[54]. Indeed, upregulation of SOCS-3 protein has also been implicated in the degradation of IRSs induced by HCV[55] and could be the link between insulin resistance and poor response to antiviral therapy due to the modulation of IFN signaling[56].
As IRSs are critical molecules involved in the transduction of insulin signal downstream IR, their degradation impairs the downstream AKT signaling pathway, a key effector of insulin action in the liver, leading to insulin resistance[53]. Indeed, this Ser/Thr kinase phosphorylates Foxo1, precluding its entry into the nucleus and, consequently, the transcription of gluconeogenic genes such as glucose 6 phosphatase (G6P) and phosphoenolpyruvate carboxykinase (PEPCK)[57]. In this manner, insulin negatively regulates hepatic glucose production. Accordingly, the negative modulation in the AKT-driven insulin signaling induced by HCV infection triggers the upregulation of gluconeogenic enzymes, G6P, and PEPCK, increasing hepatic glucose production[58]. On the other hand, activated AKT induces the phosphorylation of glycogen synthase kinase 3 (GSK3), inactivating its kinase activity, which subsequently leads to an activation of glycogen synthase, promoting glycogen synthesis[59]. Thus, insulin-induced GSK3 phosphorylation was reversed by the expression of HCV in hepatocytes, followed by the inhibition of glycogen synthesis, favoring the exacerbated hepatic glucose production observed upon HCV infection[60].
Likewise, HCV induces the expression of negative modulators of the insulin signaling pathway such as the protein tyrosine phosphatase 1B[61], the phosphatase and tensin homolog[62], and the protein phosphatase 2A[63,64]. Eventually, these phosphatases also impair the AKT pathway, negatively regulating insulin’s metabolic signaling[50].
Regarding the direct effects of the different treatments on HCV-induced impairment of insulin signaling cascade, Kawaguchi et al.[25] first demonstrated that clearance of HCV improved hepatic expression of both IRS1 and IRS2 and systemic insulin resistance in biopsy-proven HCV-infected patients after antiviral therapy based on IFN. Moreover, experimental data show that curing infected cells with IFN treatment partly modified surrogate markers of insulin resistance such as the upregulated gluconeogenesis. In this regard, while one study only has described that IFN blocked G6P-increase in HCV cells[65], another study went further, showing that the elevation of PEPCK and G6P expression as well as the enhanced glucose production were canceled upon IFN treatment in infected hepatocytes[58].
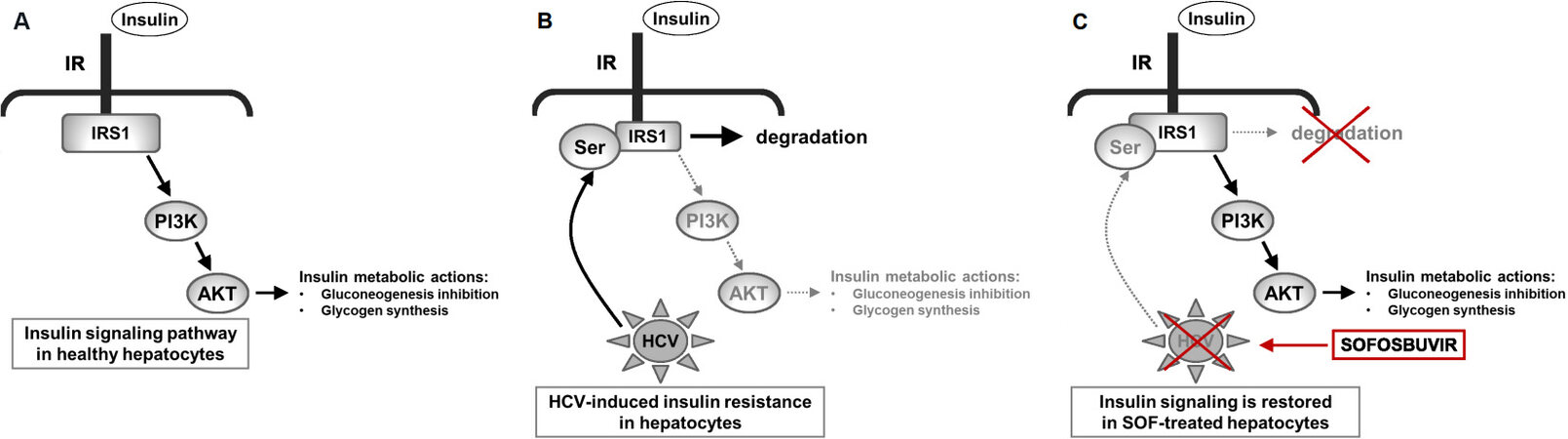
Noteworthy, we recently reported the reversion of the impaired IR/IRS1/AKT signaling pathway in HCV-infected hepatocytes cured by SOF [Figure 1]. In particular, besides the recovery of IRS1 expression, we observed that the insulin responsiveness, measured by IR tyrosine phosphorylation and AKT phosphorylation, was greatly improved in HCV-cured hepatocytes after SOF treatment compared to untreated HCV-infected cells. In addition, downstream from AKT, the SOF challenge also enhanced the phosphorylation of both Foxo1 and GSK3 upon insulin stimulation and, accordingly, triggered a decrease in gluconeogenesis and a recovery of glycogen synthesis[39].
Figure 1. Molecular effects of SOF on impaired insulin response induced by HCV infection. Schematic diagram showing how SOF improves HCV-induced insulin resistance in infected hepatocytes. (A) Critical nodes of insulin signaling implicated in insulin metabolic actions in healthy hepatocytes. (B) HCV induces serine phosphorylation of IRS1, blocking its tyrosine phosphorylation and targets IRS1 for proteosomal degradation. As IRS1 is a critical molecule involved in the transduction of insulin signal from the insulin receptor (IR), its degradation impairs the downstream AKT signalling pathway leading to insulin resistance. (C) SOF reduces IRS1 serine phosphorylation, avoiding its degradation, and further increases its protein content IRS1 in HCV-hepatocytes, recovering insulin signaling through IR/IRS1/PI3K/AKT. SOF: Sofosbuvir; HCV: hepatitis C virus; IRS: insulin receptor substrate.
Regarding clinical data, only one study has convincingly demonstrated, by using a two-step hyperinsulinemic euglycemic clamp, that hepatic insulin sensitivity improved significantly in HCV patients who achieved an SVR with either IFN-based or IFN-free DAAs regimens[66].
EFFECT OF HEPATITIS C VIRUS ON PANCREATIC BETA CELLS
The liver is the primary organ affected by HCV infection, but the presence of HCV has also been identified in the pancreas. The pancreatic beta cell function is consistently decreased in HCV patients[67]. Indeed, HCV infection impairs pancreatic beta cell function by its replication in islets and reduces insulin secretion by inhibiting exocytosis of insulin secretion. In HCV-infected cells, HCV proteins increase endoplasmic reticulum stress and activate apoptotic protease 3, inducing pancreatic beta cell death via a caspase 3-dependent pathway, leading to a decrease in beta-cell islets mass[47,68]. On the other hand, there is a general distortion of the Golgi compartment and alteration of protein kinase D activation, affecting insulin secretion in HCV-infected beta cells[67].
CONCLUSION
Chronic HCV infection is a systemic disease causing a wide range of extrahepatic complications including glucose metabolism alterations such as insulin resistance and T2DM. Available clinical evidence indicates that these metabolic complications are associated with deleterious outcomes of chronic HCV infection, such as cirrhosis and hepatocarcinoma, and it is noteworthy that HCV eradication by DAA therapy improves not only liver disease but also insulin sensitivity and glucose homeostasis. As detailed above, HCV major proteins disrupt the insulin signaling pathway at different levels leading to insulin resistance. Interestingly, SOF, a well-known DAA, is able to reverse the HCV-induced insulin resistance and improve other glucose metabolic pathways as well, suggesting that DAAs per se may have beneficial effects on glucose homeostasis. Further studies, however, are needed to elucidate the complex network of molecular mechanisms underlying the glucose metabolism disturbances linked to chronic HCV infection and how therapy with DAAs influences metabolic outcomes in cured HCV patients.
DECLARATIONS
Authors’ contributionsOrganized review structure: González-Rodríguez Á, García-Monzón C
Participated in the bibliographic search: Solís-Muñoz P, Berlana Á, del Pozo-Maroto E, Domínguez-Alcón L, Elbouayadi L, González-Rodríguez Á
Wrote the manuscript: Solís-Muñoz P, Berlana Á, González-Rodríguez Á, García-Monzón C
All authors were involved in editing the paper and had final approval of the submitted and published versions.
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by grants “Becas Gilead Sciences para proyectos de Microeliminación en Hepatitis C” from Gilead Science to CGM and grants CP14/00181 and CP19/00032 from Instituto de Salud Carlos III (ISCIII, Spain) and Fondo Europeo para el Desarrollo Regional (FEDER), and CIBERDEM (ISCIII) to González-Rodríguez Á.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022
REFERENCES
1. Global hepatitis report 2017: web Annex B: WHO estimates of the prevalence and incidence of hepatitis C virus infection by WHO region, 2015. Available from: https://apps.who.int/iris/handle/10665/277005 [Last accessed on 14 Mar 2022].
2. Borgia SM, Hedskog C, Parhy B, et al. Identification of a novel hepatitis C virus genotype from Punjab, India: expanding classification of hepatitis C virus into 8 genotypes. J Infect Dis 2018;218:1722-9.
3. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61:S45-57.
4. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998;352:1426-32.
5. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-82.
6. Hézode C. Boceprevir and telaprevir for the treatment of chronic hepatitis C: safety management in clinical practice. Liver Int 2012;32 Suppl 1:32-8.
7. Leuw P, Stephan C. Protease inhibitors for the treatment of hepatitis C virus infection. GMS Infect Dis 2017;5:Doc08.
8. Herbst DA Jr, Reddy KR. Sofosbuvir, a nucleotide polymerase inhibitor, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs 2013;22:527-36.
9. Rodriguez-Torres M, Lawitz E, Kowdley KV, et al. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol 2013;58:663-8.
10. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-87.
11. Sulkowski MS, Naggie S, Lalezari J, et al. PHOTON-1 Investigators. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014;312:353-61.
12. Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-Pibrentasvir for 8 or 12 weeks in HCV Genotype 1 or 3 infection. N Engl J Med 2018;378:354-69.
13. Ryu JK, Lee SB, Hong SJ, Lee S. Association of chronic hepatitis C virus infection and diabetes mellitus in Korean patients. Korean J Intern Med 2001;16:18-23.
14. Skowroński M, Zozulińska D, Juszczyk J, Wierusz-Wysocka B. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care 2006;29:750; author reply 751.
15. Ahmed N, Rashid A, Naveed AK, Bashir Q. Effect of HCV on fasting glucose, fasting insulin and peripheral insulin resistance in first 5 years of infection. J Pak Med Assoc 2016;66:140-2.
16. Chaudhari R, Fouda S, Sainu A, Pappachan JM. Metabolic complications of hepatitis C virus infection. World J Gastroenterol 2021;27:1267-82.
17. Gastaldi G, Goossens N, Clément S, Negro F. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res 2017;8:149-59.
18. Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther 2020;51:216-30.
19. Ambachew S, Eshetie S, Geremew D, Endalamaw A, Melku M. Prevalence of type 2 diabetes mellitus among hepatitis C virus-infected patients: a protocol for systematic review and meta-analysis. Syst Rev 2019;8:60.
20. Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol 1994;21:1135-9.
21. Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003;38:50-6.
22. Cacoub P, Carrat F, Bédossa P, et al. Insulin resistance impairs sustained virological response rate to pegylated interferon plus ribavirin in HIV-hepatitis C virus-coinfected patients: HOMAVIC-ANRS HC02 Study. Antivir Ther 2009;14:839-45.
23. El-Zayadi AR, Anis M. Hepatitis C virus induced insulin resistance impairs response to anti viral therapy. World J Gastroenterol 2012;18:212-24.
24. Romero-Gómez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005;128:636-41.
25. Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol 2007;102:570-6.
26. Khattab MA, Eslam M, Shatat M, et al. Changes in adipocytokines and insulin sensitivity during and after antiviral therapy for hepatitis C genotype 4. J Gastrointestin Liver Dis 2012;21:59-65.
27. Delgado-Borrego A, Jordan SH, Negre B, et al. Halt-C Trial Group. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:458-62.
28. Thompson AJ, Patel K, Chuang WL, et al. ACHIEVE-1 and ACHIEVE-2/3 Study Teams. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut 2012;61:128-34.
29. Chien CH, Lin CL, Hu CC, Chang JJ, Chien RN. Clearance of hepatitis C virus improves insulin resistance during and after peginterferon and ribavirin therapy. J Interferon Cytokine Res 2015;35:981-9.
30. Lo Iacono O, Venezia G, Petta S, et al. The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther 2007;25:1181-91.
31. Aghemo A, Prati GM, Rumi MG, et al. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology 2012;56:1681-7.
32. Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 2009;49:739-44.
33. Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol 2008;48:721-7.
34. Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care 2006;29:2462-6.
35. Boraie MB, Elnaggar YA, Ahmed MO, Mahmoud AM. Effect of direct acting antiviral therapy of Chronic Hepatitis C virus on insulin resistance and Type2 DM in Egyptian patients (prospective study). Diabetes Metab Syndr 2019;13:2641-6.
36. Ciancio A, Bosio R, Bo S, et al. Significant improvement of glycemic control in diabetic patients with HCV infection responding to direct-acting antiviral agents. J Med Virol 2018;90:320-7.
37. Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care 2017;40:1173-80.
38. Zied HY, Abo Alnasr NM, El-Bendary AS, Abd-Elsalam S, Hagag RY. Effect of treatment with direct antiviral agents (DAAs) on glycemic control in patients with type 2 diabetes mellitus & hepatitis C virus genotype 4. Diabetes Metab Syndr 2020;14:679-82.
39. Rey E, Ampuero J, Molina-Jiménez F, et al. Sofosbuvir improves HCV-induced insulin resistance by blocking IRS1 degradation. Clin Transl Med 2021;11:e275.
40. Adinolfi LE, Petta S, Fracanzani AL, et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: a prospective study. Diabetes Obes Metab 2020;22:2408-16.
41. Tada T, Kumada T, Toyoda H, et al. Viral eradication reduces both liver stiffness and steatosis in patients with chronic hepatitis C virus infection who received direct-acting anti-viral therapy. Aliment Pharmacol Ther 2018;47:1012-22.
42. Nevola R, Rinaldi L, Zeni L, et al. Metabolic and renal changes in patients with chronic hepatitis C infection after hepatitis C virus clearance by direct-acting antivirals. JGH Open 2020;4:713-21.
43. Carvalho JR, Velosa J, Serejo F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication - comparison of the new direct-acting antiviral agents with the old regimens. Scand J Gastroenterol 2018;53:857-63.
44. Russo FP, Zanetto A, Gambato M, et al. Hepatitis C virus eradication with direct-acting antiviral improves insulin resistance. J Viral Hepat 2020;27:188-94.
45. Ribaldone DG, Sacco M, Saracco GM. The effect of viral clearance achieved by direct-acting antiviral agents on hepatitis C virus positive patients with type 2 diabetes mellitus: a word of caution after the initial enthusiasm. J Clin Med 2020;9:563.
46. Sacco M, Saracco GM. The impact of direct-acting antiviral treatment on glycemic homeostasis in patients with chronic hepatitis C. Minerva Gastroenterol (Torino) 2021;67:264-72.
47. Mastrototaro L, Roden M. Insulin resistance and insulin sensitizing agents. Metabolism 2021;125:154892.
48. Bandiera S, Pernot S, El Saghire H, et al. Hepatitis C virus-induced upregulation of microRNA miR-146a-5p in hepatocytes promotes viral infection and deregulates metabolic pathways associated with liver disease pathogenesis. J Virol 2016;90:6387-400.
49. Klover PJ, Mooney RA. Hepatocytes: critical for glucose homeostasis. Int J Biochem Cell Biol 2004;36:753-8.
50. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006;7:85-96.
51. Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol 2007;47:142-56.
52. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012;55:2565-82.
53. Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol 2008;82:2606-12.
54. García-Monzón C, Lo Iacono O, Mayoral R, et al. Hepatic insulin resistance is associated with increased apoptosis and fibrogenesis in nonalcoholic steatohepatitis and chronic hepatitis C. J Hepatol 2011;54:142-52.
55. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol 2004;165:1499-508.
56. Persico M, Capasso M, Persico E, et al. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology 2007;46:1009-15.
57. Zhang W, Patil S, Chauhan B, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 2006;281:10105-17.
58. Deng L, Shoji I, Ogawa W, et al. Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J Virol 2011;85:8556-68.
59. Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract 2007;77 Suppl 1:S49-57.
60. Hsieh MJ, Lan KP, Liu HY, et al. Hepatitis C virus E2 protein involve in insulin resistance through an impairment of Akt/PKB and GSK3β signaling in hepatocytes. BMC Gastroenterol 2012;12:74.
61. Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Molecular Cell 2000;6:1401-12.
62. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998;273:13375-8.
63. Ivaska J, Nissinen L, Immonen N, Eriksson JE, Kähäri VM, Heino J. Integrin alpha 2 beta 1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3 beta. Mol Cell Biol 2002;22:1352-9.
64. Duong FH, Filipowicz M, Tripodi M, La Monica N, Heim MH. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology 2004;126:263-77.
65. Shlomai A, Rechtman MM, Burdelova EO, et al. The metabolic regulator PGC-1α links hepatitis C virus infection to hepatic insulin resistance. J Hepatol 2012;57:867-73.
66. Lim TR, Hazlehurst JM, Oprescu AI, et al. Hepatitis C virus infection is associated with hepatic and adipose tissue insulin resistance that improves after viral cure. Clin Endocrinol (Oxf) 2019;90:440-8.
67. Chen J, Wang F, Zhou Y, et al. Chronic hepatitis C virus infection impairs insulin secretion by regulation of p38δ MAPK-dependent exocytosis in pancreatic β-cells. Clinical Science 2020;134:529-42.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Solís-Muñoz P, Berlana, Pozo-Maroto E, Domínguez-Alcón L, Elbouayadi L, García-Monzón C, González-Rodríguez. Insights on the disruption of glucose metabolism and hepatic insulin resistance induced by hepatitis C virus. Metab Target Organ Damage 2022;2:5. http://dx.doi.org/10.20517/mtod.2021.18
AMA Style
Solís-Muñoz P, Berlana, Pozo-Maroto E, Domínguez-Alcón L, Elbouayadi L, García-Monzón C, González-Rodríguez. Insights on the disruption of glucose metabolism and hepatic insulin resistance induced by hepatitis C virus. Metabolism and Target Organ Damage. 2022; 2(2): 5. http://dx.doi.org/10.20517/mtod.2021.18
Chicago/Turabian Style
Solís-Muñoz, Pablo, Ángela Berlana, Elvira del Pozo-Maroto, Lucía Domínguez-Alcón, Liliam Elbouayadi, Carmelo García-Monzón, Águeda González-Rodríguez. 2022. "Insights on the disruption of glucose metabolism and hepatic insulin resistance induced by hepatitis C virus" Metabolism and Target Organ Damage. 2, no.2: 5. http://dx.doi.org/10.20517/mtod.2021.18
ACS Style
Solís-Muñoz, P.; Berlana.; Pozo-Maroto E.; Domínguez-Alcón L.; Elbouayadi L.; García-Monzón C.; González-Rodríguez. Insights on the disruption of glucose metabolism and hepatic insulin resistance induced by hepatitis C virus. Metab Target Organ Damage. 2022, 2, 5. http://dx.doi.org/10.20517/mtod.2021.18
About This Article
Copyright
Data & Comments
Data

 Cite This Article 17 clicks
Cite This Article 17 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.