From liver fat to full-blown metabolic disorder: the kidney as target organ
In 1980, Ludwig et al. identified a form of liver disease characterized by accumulation of fat in the form of triglycerides within the hepatocytes, very similar to what occurs in response to alcohol intake, but in subjects with no alcohol abuse[1]. The condition was named nonalcholic fatty liver disease (NAFLD) and was initially considered a trivial occurrence, with scarce or no clinical significance. In only 50 years, NAFLD has climbed up the list of most common liver diseases; it is present in over 25% of the total adult population[2], is the second and most rapidly growing indication for liver transplantation[3,4], and is projected to become the most common cause of liver cancer in Western countries[5]. How could this have happened?
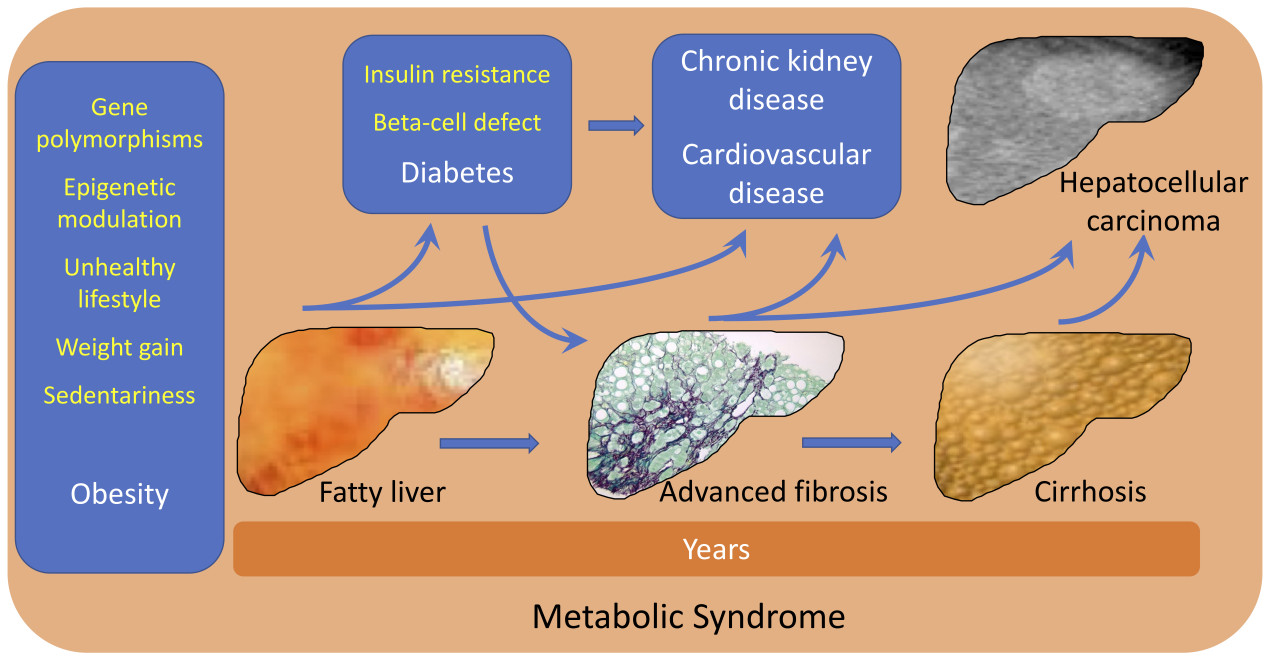
The accumulation of fat in the liver depends on an imbalance between fat storage and fat disposal, leading to triglyceride spillover in non-adipose tissues, not limited to the liver, but also including the pancreas, the heart, and the vascular tree. Although a few genetic determinants and epigenetic modifiers have been identified, total body and particularly visceral fat accumulation remain the leading cause. The world prevalence of obesity (body mass index ≥ 30 kg/m2 or equivalent values for subjects of Asian origin) trebled in the period from 1975 to 2016 (from 4.3% to 13.2%); it was over 30% in the United States and several Arab countries and reached 50%-60% in the Pacific Islands, accompanied by another 25% in the range of overweight[6,7]. The obesity epidemic has been associated with a parallel increase in the prevalence of diabetes, with over 400 million patients around the world expected to suffer from the multi-morbidities associated with diabetes and sharing diabetes outcomes[8]. Obesity (70%-90% with NAFLD) and type 2 diabetes (around 50%-60%) are natural reservoirs of NAFLD, thus contributing to NAFLD’s increase and final cardiovascular events. Indeed, cardiovascular events are the most frequent cause of death in NAFLD, much more common than death from liver disease.
In summary, there is ample evidence that NAFLD is intimately associated with metabolic syndrome, as initially demonstrated about 20 years ago[9], which has led to considering NAFLD the hepatic component of the metabolic syndrome[10].
However, liver disease remains a pivotal factor in whole body metabolic imbalance. A recent meta-analysis suggests that whole body metabolic dysregulations do not only mirror comorbidities. Mantovani et al., in a reappraisal of the risk of incident chronic kidney disease (CKD) associated with NAFLD, reported data on over 1.2 million subjects with normal renal function entered into 13 observational studies in different countries (28.1% with NAFLD identified by blood biomarkers, imaging, International Classification of Disease codes, or liver biopsy)[11]. During an average follow-up of 9.7 years in 10 studies where control subjects were available, the risk of developing CKD stage ≥ 3 (glomerular filtration rate (GFR) < 60 mL/min per 1.73 m2, with/without proteinuria) was nearly 50% higher in the presence of NAFLD [hazard ratio (HR): 1.43; 95% confidence interval (CI): 1.33-1.64], and the data were consistent across methods of NAFLD diagnosis, geographic area, duration of follow-up, and risks of bias[11].
These results were largely expected, based on the above considerations, and had been previously demonstrated in two limited meta-analyses[12,13]. However, two points merit particular discussion.
First, the risk of incident CKD is maintained after adjustment for age, sex, smoking, BMI, pre-existing diabetes, hypertension, and other components of metabolic syndrome; these factors had already been adjusted for in the original studies and the meta-regression did not change the results. This points to a specific effect of liver disease, but no study included incident diabetes or hypertension as additional cofactors. In other words, it is possible that part of the risk of incident CKD might stem from the ongoing progressive accumulation of comorbidities.
Whatever the pathogenic mechanism, the role of the liver should not be disregarded. Taylor et al. first postulated a primary role of liver fat accumulation in the pathogenesis of insulin resistance and type 2 diabetes[14], exacerbated by the defective insulin response associated with pancreatic fat accumulation[15]. These events are also the basis for diabetes remission by calorie restriction, as demonstrated in the DIRECT program of weight reduction by low calorie intake[16,17].
Secondly, there is ample evidence that CKD may be considered a surrogate measure of cardiovascular risk: from the Kaiser Permanente Analysis of over one million individuals, we know that CKD stage increases the risk of cardiovascular events and cardiovascular death (not to mention all-cause death)[18]. Considering the high rate of cardiovascular events in NAFLD, it would be extremely interesting to define the intertwined relationship between cardiovascular and renal disease in NAFLD. Although end-stage renal disease is a possible consequence of advanced liver disease, in most cases with NAFLD progressive CKD is expected to be the effect of extensive atherosclerotic disease.
The role of severity of liver disease in CKD progression was indeed among the scopes of the updated meta-analysis, but data could only be retrieved from a limited proportion of studies without comparator controls. Under these circumstances, the authors opted for a descriptive analysis. The results show a higher risk in a study of subjects classified as advanced fibrosis by biomarker [high nonalcholic fibrosis score (NFD) vs. low NFD; HR, 1.59], as well as in two studies where liver biopsy was available (fibrosis ≥ F3 vs. fibrosis < F3; HR, 2.75 and 3.25, respectively). The authors concluded that an effect of disease severity is very likely, but other data are needed to support this evidence.
Many more data might soon be retrieved, considering that large databases should soon be available following recommendations from the guidelines to systematically identify NAFLD patients with advanced fibrosis[19,20], which are also endorsed by patient associations[21]. However, a problem remains: how is it possible to bend the curve of NAFLD prevalence and severity? As with many non-communicable diseases, NAFLD is an orphan of approved drug treatment and interventions are limited towards motivation and counseling for a healthy lifestyle (dietary restriction and habitual physical activity). In addition, referral to nephrologists to reduce progressive kidney failure, as advocated by the authors of the meta-analysis[11], is largely dependent on adherence to lifestyle, coupled with the exclusion of drugs at risk of kidney damage. However, new opportunities are on the horizon. Both sodium-glucose cotransporter-2 inhibitors (SGLT-2Is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been shown to reduce progressive renal failure in patients with/without diabetes[22], thus directly reducing the risk outlined in the recent meta-analysis[11]. However, both GLP-1RAs and dual GLP-1/GIP RAs also have an impressive activity on weight loss. The recent studies testing high-dose semaglutide (STEP program) and tirzepatide (SURMONT-1 study) in the treatment of obesity showed that these drugs are able to produce an average weight loss of 15%-20% of initial body weight in 68-72 weeks[23,24]. These results mimic the effects of bariatric surgery and might help correct obesity and its long-term consequences, including renal outcomes, a hidden consequence of the obesity epidemic[25]. When coupled with adequate lifestyle interventions[26], they might also cure NAFLD from its initial appearance in the stage of steatosis, thus limiting progression to fibrosis, cirrhosis, and eventually hepatocellular carcinoma. We are facing a new era in the drug treatment of obesity, with hopes to bend the curve of metabolic syndrome.
DECLARATIONS
Authors’ contributionsJointly discussed the editorial: Petroni LM, Marchesini G
Searched the literature: Petroni LM
Drafted the manuscript: Marchesini G
Both authors approved the final version.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestNot applicable.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experience with an hitherto unnamed disease. Mayo Clin Proc 1980;55:434-8.
2. Karlsen TH, Sheron N, Zelber-sagi S, et al. The EASL–Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. The Lancet 2022;399:61-116.
3. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547-55.
4. Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580-589.
5. Vitale A, Svegliati-Baroni G, Ortolani A, et al. Italian Liver Cancer (ITA.LI.CA) group. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002-2033: the ITA.LI.CA database. Gut 2022;gutjnl-2021-324915.
6. Our World in Data. Obesity 2022. Available from: https://ourworldindata.org/obesity [Last accessed on 4 Jul 2022].
7. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021. Available from: https://suckhoenoitiet.vn/download/Atla-benh-dai-thao-duong-2-1511669800.pdf [Last accessed on 4 Jul 2022].
8. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. The Lancet 2020;396:1223-49.
9. Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844-50.
10. Yki-järvinen H. nonalcholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014;2:901-10.
11. Mantovani A, Petracca G, Beatrice G, et al. nonalcholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut 2022;71:156-62.
12. Musso G, Gambino R, Tabibian JH, et al. Association of nonalcholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11:e1001680.
13. Mantovani A, Zaza G, Byrne CD, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism 2018;79:64-76.
14. Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008;51:1781-9.
15. Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007;30:2916-21.
16. Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab 2018;28:547-556.
17. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. The Lancet 2018;391:541-51.
18. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305.
19. Association for the Study of the Liver (EASL)., European Association for the Study of Diabetes (EASD)., European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of nonalcholic fatty liver disease. Obes Facts 2016;9:65-90.
20. Italiana per lo Studio del Fegato (AISF), Società Italiana di Diabetologia (SID) and Società Italiana dell’Obesità (SIO)., Members of the guidelines panel., Coordinator., AISF Members., SID Members., SIO Members., Metodologists. nonalcholic fatty liver disease in adults 2021: a clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Dig Liver Dis 2022;54:170-82.
21. Francque SM, Marchesini G, Kautz A, et al. nonalcholic fatty liver disease: a patient guideline. JHEP Rep 2021;3:100322.
22. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2022;376:o109.
23. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med ; doi: 10.1056/NEJMoa2206038.
24. Wilding JPH, Batterham RL, Calanna S, et al. STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989-1002.
25. Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. J Bras Nefrol 2017;39:1-10.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Petroni ML, Marchesini G. From liver fat to full-blown metabolic disorder: the kidney as target organ. Metab Target Organ Damage 2022;2:10. http://dx.doi.org/10.20517/mtod.2022.17
AMA Style
Petroni ML, Marchesini G. From liver fat to full-blown metabolic disorder: the kidney as target organ. Metabolism and Target Organ Damage. 2022; 2(3): 10. http://dx.doi.org/10.20517/mtod.2022.17
Chicago/Turabian Style
Petroni, Maria L., Giulio Marchesini. 2022. "From liver fat to full-blown metabolic disorder: the kidney as target organ" Metabolism and Target Organ Damage. 2, no.3: 10. http://dx.doi.org/10.20517/mtod.2022.17
ACS Style
Petroni, ML.; Marchesini G. From liver fat to full-blown metabolic disorder: the kidney as target organ. Metab Target Organ Damage. 2022, 2, 10. http://dx.doi.org/10.20517/mtod.2022.17
About This Article
Copyright
Data & Comments
Data

 Cite This Article 19 clicks
Cite This Article 19 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.