Assessing strategies to target screening for advanced liver fibrosis among overweight and obese patients
Abstract
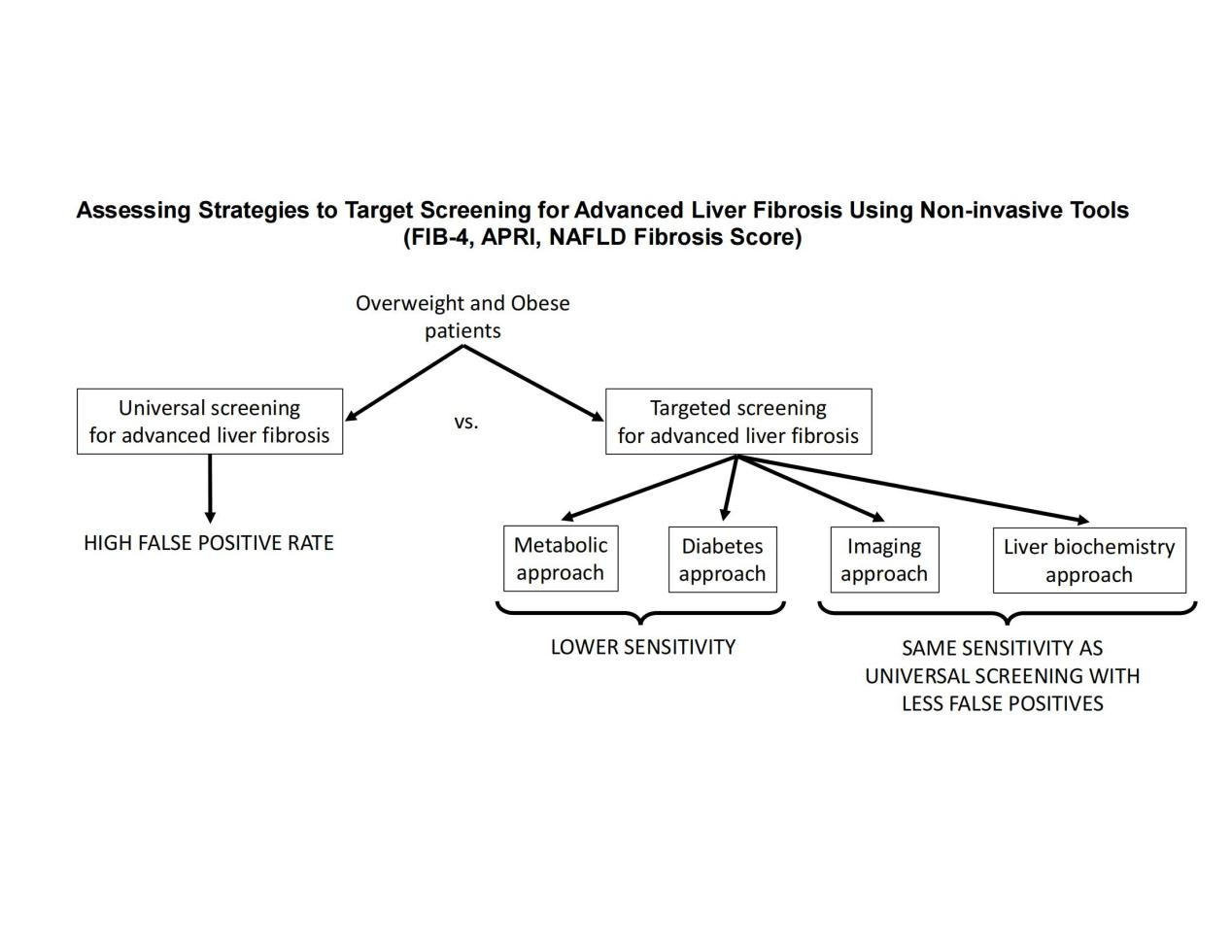
Aim: The optimal screening strategy for advanced liver fibrosis in overweight and obese patients is unknown. The aim of this study is to compare the performance of different strategies to select patients at high risk of advanced liver fibrosis for screening using non-invasive tools.
Methods: All patients underwent: liver 1H-MRS and percutaneous liver biopsy (in those with nonalcoholic fatty liver disease [NAFLD]). Unique selection strategies were compared to determine the best screening algorithm: (A) A "metabolic approach": selecting patients based on HOMA-IR ≥ 3; (B) A "diabetes approach": selecting only patients with type 2 diabetes; (C) An "imaging approach": selecting patients with hepatic steatosis based on 1H-MRS; (D) A "liver biochemistry approach": selecting patients with elevated ALT (i.e., ≥ 30 IU/L for males and ≥ 19 IU/L for females); and (E) Universal screening of overweight and obese patients. FIB-4 index, NAFLD fibrosis score, and APRI were applied as screening strategies.
Results: A total of 275 patients were included in the study. Patients with advanced fibrosis (n = 29) were matched for age, gender, ethnicity, and BMI. Selecting patients by ALT elevation provided the most effective strategy, limiting the false positive rate while maintaining the sensitivity compared to universal screening. Selecting patients by any other strategy did not contribute to increasing the sensitivity of the approach and resulted in more false positive results.
Conclusion: Universal screening of overweight/obese patients for advanced fibrosis with non-invasive tools is unwarranted, as selection strategies based on elevated ALT levels lead to the same sensitivity with a lower false positive rate (i.e., fewer patients that would require a liver biopsy or referral to hepatology).
Keywords
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has become a serious public health problem with an estimated prevalence of ~25% in the overall population[1]. Among overweight or obese patients, this prevalence is probably much higher in the range of 50%-60%[2,3]. Despite this high prevalence, there is still no established algorithm to screen for liver disease in these patients. A percutaneous liver biopsy remains the gold standard for the diagnosis of nonalcoholic steatohepatitis (NASH) and for determining the liver fibrosis stage[4]. Numerous non-invasive tools, including plasma biomarkers, imaging tests, and clinical scores combining demographic and biochemical data, have been proposed[5,6]. They have been widely used to predict the presence of NAFLD, NASH, and/or advanced fibrosis. Moreover, national and international guidelines have even endorsed some of these approaches[4,7,8]. However, a clear consensus regarding which patients would benefit the most from advanced fibrosis screening is lacking. Specifically, it is unclear whether all overweight or obese patients should be screened for hepatic fibrosis, or if screening should be reserved only for selected patients at high risk of liver fibrosis. In which case, the best strategy to select these high-risk patients is currently unknown. While different approaches have been proposed (i.e., selecting patients based on the presence of diabetes, presence of metabolic syndrome, elevated plasma aminotransferases, or presence of hepatic steatosis)[8], no prior study has compared these approaches head-to-head. Therefore, the aim of this study is to compare different strategies to target screening for advanced liver fibrosis in overweight and obese patients.
METHODS
Research subjects
Patients included in this study were recruited from hepatology and endocrinology clinics at the University of Florida in Gainesville, FL and the University of Texas Health Science Center at San Antonio (UTHSCSA) in San Antonio, TX, as well as from the general population. Patients included in this manuscript were previously included in other manuscripts assessing the metabolic implications of NAFLD and NASH or assessing therapies for NASH[9-11]. Adult overweight or obese (BMI ≥ 25 kg/m2) patients were included in the study after exclusion of secondary liver diseases (hepatitis B or C, autoimmune hepatitis, hemochromatosis, Wilson’s disease, drug-induced hepatitis, etc.), significant alcohol consumption (≥ 30 gm/day for males and
Study design
In this cross-sectional study, patients underwent a liver 1H-MRS, a percutaneous liver biopsy (if diagnosed with NAFLD by imaging), and baseline measurements of all components of fibrosis-4 index (FIB-4), NAFLD fibrosis score (NFS) and AST to platelet ratio index (APRI). Patients with incomplete data were excluded from the study. Universal screening for advanced fibrosis in overweight and obese patients was compared against different strategies to select a high-risk subpopulation based on typical information available in the clinical setting. The different approaches aimed to target screening for advanced fibrosis were: (A) A "metabolic approach": selecting only patients with elevated insulin resistance based on HOMA-IR ≥ 3; (B) A "diabetes approach": selecting only patients with T2D; (C) A "imaging approach": selecting patients with known hepatic steatosis based on 1H-MRS with intrahepatic triglyceride content ≥ 5.6%; (D) A "liver biochemistry approach": selecting patients with elevated ALT (i.e., ≥ 30 IU/L for males and ≥ 19 IU/L for females). These were compared to a "universal screening approach": all overweight and obese patients were screened. Head-to-head comparisons were performed among different diagnostic strategies to assess their accuracy in detecting patients with advanced fibrosis and the number of biopsies needed for that. For the purposes of this study, the assumption was that any patient with a positive screening would undergo a liver biopsy.
Intrahepatic triglyceride content and histology
Intrahepatic triglyceride accumulation was measured by liver 1H-MRS as previously described[12]. The intrahepatic triglyceride content of ≥ 5.6% was considered diagnostic of NAFLD[4,13]. Patients without NAFLD were considered as not having advanced fibrosis for the purpose of this study. Percutaneous liver biopsies were performed with US guidance in those with NAFLD considered at high risk for progressive liver disease. All biopsies were blindly read by the same pathologist, who was unaware of the patient’s characteristics. Diagnosis of NASH was made following standard criteria[14]: the presence of zone 3 macrovesicular steatosis (any grade), hepatocellular ballooning (any degree), and lobular inflammatory infiltrate (any amount). Fibrosis was classified based on standard criteria[15]. Advanced fibrosis was defined as the presence of fibrosis stages 3 or 4 (F3 or F4). Clinically significant fibrosis (or moderate fibrosis) was defined as fibrosis stages 2 or higher (i.e., ≥ F2). NAFLD activity score (NAS) was calculated as the sum of the steatosis, inflammation, and ballooning grades in the liver biopsy. The mean length of liver biopsies was 1.6 cm (95%CI: 1.5-1.7 cm).
Non-invasive scores used to diagnose advanced fibrosis
Based on simplicity and widespread availability, NFS, FIB-4, and APRI were used as non-invasive biomarkers for the prediction of advanced fibrosis. Their formulas are public domain and can be found elsewhere[16].
Statistical analysis
Data has been summarized as number (percentage) for categorical variables and mean ± SD for continuous variables. Sensitivity, specificity, and positive and negative predictive values for the different approaches were calculated using histology as the gold standard for the diagnosis of advanced fibrosis. A two-tailed value of P < 0.05 was considered to indicate statistical significance. Analyses were performed with Stata 11.1 (StataCorp LP, College Station, TX) and graphs with Prism 6.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Patients’ characteristics
A total of 275 overweight and obese patients were included in the study. The proportion of patients with NAFLD was 67% [Supplementary Table 1]. Patients’ characteristics have been summarized in Table 1, where patients were divided based on the presence or absence of advanced fibrosis in the liver biopsy. As can be observed, groups were well-matched for important clinical and anthropometric characteristics, such as age, gender, ethnicity, weight, and BMI. Patients with advanced fibrosis had a higher prevalence of type 2 diabetes and a consequent higher fasting plasma glucose and hemoglobin A1c. As expected, patients with advanced fibrosis also showed higher levels of plasma AST and ALT; however, AST/ALT ratio was not different compared to patients without advanced fibrosis (0.92 ± 0.27 vs. 0.88 ± 0.31, P = 0.54). All histological parameters were also worse in patients with advanced fibrosis, except for steatosis, which was technically not significantly different between both groups (P = 0.06). However, intrahepatic triglyceride content based on liver 1H-MRS was significantly higher in patients with advanced fibrosis.
Patients’ characteristics based on the presence of advanced fibrosis (F3 or F4)
| Patients without advanced fibrosis (n = 246) | Patients with advanced fibrosis (n = 29) | P value | |
| Age, years | 54 ± 11 | 54 ± 9 | 0.99 |
| Gender, male/female % | 74%/26% | 72%/28% | 0.86 |
| Ethnicity | 0.54 | ||
| Caucasian, % | 46% | 45% | |
| Hispanic, % | 37% | 48% | |
| African-American, % | 15% | 7% | |
| Other, % | 2% | - | |
| Weight, kg | 99 ± 17 | 101 ± 16 | 0.59 |
| Body mass index, kg/m2 | 33.8 ± 4.9 | 35.1 ± 5.3 | 0.20 |
| Presence of diabetes, % | 59% | 86% | 0.004 |
| Diabetes medications | |||
| Metformin, % | 41% | 72% | 0.001 |
| Sulfonylureas, % | 22% | 48% | 0.003 |
| Insulin, % | 12% | 34% | 0.003 |
| Statin use, % | 58% | 59% | 0.92 |
| Blood pressure medication use, % | 69% | 83% | 0.12 |
| A1c, % | 6.4 ± 1.1 | 7.4 ± 1.3 | < 0.001 |
| Fasting plasma glucose, mg/ml | 125 ± 34 | 147 ± 41 | 0.002 |
| Intrahepatic triglyceride content, % | 11 ± 10 | 15 ± 7 | 0.037 |
| Aspartate aminotransferase, U/L | 32 ± 19 | 63 ± 37 | < 0.001 |
| Alanine aminotransferase, U/L | 43 ± 34 | 73 ± 44 | 0.005 |
| NAFLD activity score | 3.1 ± 1.5 | 5.0 ± 1.3 | < 0.001 |
| Steatosis grade Patients with grade 0-1 Patients with grade 2 Patients with grade 3 | 1.5 ± 0.8 55% 31% 13% | 1.8 ± 0.6 28% 66% 7% | 0.06 |
| Inflammation grade Patients with grade 0 Patients with grade 1 Patients with grade 2-3 | 1.1 ± 0.6 11% 71% 18% | 1.7 ± 0.5 0% 34% 66% | < 0.001 |
| Ballooning grade Patients with grade 0 Patients with grade 1 Patients with grade 2 | 0.5 ± 0.6 56% 38% 6% | 1.6 ± 0.6 7% 31% 62% | < 0.001 |
| Fibrosis stage Patients with stage 0 Patients with stage 1 Patients with stage 2 Patients with stage 3-4 | 0.6 ± 0.7 52% 37% 11% 0% | 3.1 ± 0.3 0% 0% 0% 100% | < 0.001 |
Performance of the different strategies to identify patients with advanced fibrosis
The performance of different strategies to select patients at risk of advanced fibrosis was compared by applying three different, widely available, non-invasive clinical scores as the screening approach: FIB-4 index [Table 2], NAFLD fibrosis score [Table 3], and APRI [Table 4].
Performance of different strategies to identify patients with advanced fibrosis (F3 or F4) based on the FIB-4 index
| Selection Strategy | Number of patients selected | Patients with FIB-4 ≥ 1.30 | Patients identified with advanced fibrosis | Number of biopsies per patient identified with advanced fibrosis | Patients that would need a biopsy per 100 patients | Sensitivity of the approach (%) | False positive rate (%) |
| Metabolic approach HOMA-IR ≥ 3 | 129 | 67 | 20 | 3.35 | 24 | 69 | 19 |
| Diabetes approach Patients with T2D | 169 | 87 | 20 | 4.35 | 32 | 69 | 27 |
| Imaging approach NAFLD by 1H-MRS | 183 | 74 | 22 | 3.36 | 27 | 76 | 21 |
| Liver biochemistry approach ALT ≥ 30 IU/L in males and ≥ 19 IU/L in females | 168 | 70 | 22 | 3.18 | 25 | 76 | 20 |
| Universal screening All patients | 275 | 114 | 22 | 5.18 | 41 | 76 | 37 |
Performance of different strategies to identify patients with advanced fibrosis (F3 or F4) based on NFS
| Screening strategy | Number of patients selected | Patients with NFS ≥ -1.455 | Patients identified with advanced fibrosis | Number of biopsies per patient identified with advanced fibrosis | Patients that would need a biopsy per 100 patients | Sensitivity of the approach (%) | False positive rate (%) |
| Metabolic approach HOMA-IR ≥ 3 | 129 | 116 | 25 | 4.64 | 42 | 86 | 38 |
| Diabetes approach Patients with T2D | 169 | 151 | 24 | 6.29 | 56 | 83 | 53 |
| Imaging approach NAFLD by 1H-MRS | 183 | 147 | 27 | 5.44 | 54 | 93 | 50 |
| Liver biochemistry approach ALT ≥ 30 IU/L in males and ≥ 19 IU/L in females | 168 | 136 | 28 | 4.85 | 50 | 97 | 42 |
| Universal screening All patients | 275 | 229 | 28 | 8.18 | 85 | 97 | 84 |
Performance of different strategies to identify patients with advanced fibrosis (F3 or F4) based on APRI
| Screening strategy | Number of patients selected | Patients with APRI ≥ 0.50 | Patients identified with advanced fibrosis | Number of biopsies per patient identified with advanced fibrosis | Patients that would need a biopsy per 100 patients | Sensitivity of the approach (%) | False positive rate (%) |
| Metabolic approach HOMA-IR ≥ 3 | 129 | 50 | 20 | 2.50 | 18 | 69 | 12 |
| Diabetes approach Patients with T2D | 169 | 43 | 19 | 2.26 | 16 | 66 | 10 |
| Imaging approach NAFLD by 1H-MRS | 183 | 62 | 22 | 2.81 | 23 | 76 | 16 |
| Liver biochemistry approach ALT ≥ 30 IU/L in males and ≥ 19 IU/L in females | 168 | 65 | 22 | 2.95 | 24 | 76 | 17 |
| Universal screening All patients | 275 | 67 | 22 | 3.05 | 24 | 76 | 18 |
FIB-4 index
All strategies using FIB-4 index, except the ones based on measuring insulin resistance or selecting for the presence of diabetes, led to the identification of 22 of the 29 patients with advanced fibrosis (i.e., similar sensitivity of 76% across the different strategies) [Table 2]. The main differences among these strategies are related to the rate of false positives and, therefore, the potential number of biopsies that would be needed, assuming that all patients with a positive screening would be advised to undergo a percutaneous liver biopsy. Universal screening did not help to identify more patients with advanced fibrosis compared to more restrictive approaches, like the imaging approach or the liver biochemistry approach. Based on a universal approach, 41 biopsies would be needed for every 100 patients screened, which means ~5.18 biopsies performed per each patient identified with advanced fibrosis. Identification of patients based on elevated ALT was the strategy that allowed for the lowest false positive rate while maintaining sensitivity. This strategy would result in the need for 25 biopsies for every 100 patients screened, reducing to only ~3.18 biopsies needed to identify one patient with advanced fibrosis. Results for the imaging approach were very similar to the approach using elevated ALT [Table 2]. The metabolic and diabetes approaches required a higher number of biopsies compared to the liver biochemistry approach and resulted in a lower sensitivity to detect advanced fibrosis.
NAFLD fibrosis score
Applying NAFLD fibrosis score allowed for higher sensitivities compared to FIB-4 index, but significantly higher false positive rates [Table 3]. Universal screening of overweight and obese patients resulted in 229 out 275 patients with a positive screening (i.e., need for 85 biopsies for every 100 patients screened or ~8.18 biopsies to identify one patient with advanced fibrosis). The need for biopsies was reduced by selecting patients based on insulin resistance (42 biopsies per 100 screened patients), presence of diabetes (56 biopsies per 100 screened patients), presence of NAFLD (54 biopsies per 100 screened patients), or abnormal ALT (50 biopsies per 100 screened patients). The metabolic and liver biochemistry approaches were the ones with the lower number of biopsies needed to identify one patient with advanced fibrosis (4.64 and 4.85, respectively). However, the liver biochemistry approach had a higher sensitivity (97% vs. 86%). Of note, using the NAFLD fibrosis score resulted in the need for significantly more biopsies compared to the FIB-4 index, reardless of the population selection strategy.
APRI
Using APRI to screen for advanced fibrosis in overweight and obese patients resulted in sensitivities similar to FIB-4 index, but overall lower false positive rates [Table 4]. A universal screening approach with APRI allowed to identify 22 out of 29 patients with advanced fibrosis, and it resulted in the need for 67 biopsies among 275 patients (24 out of 100 patients screened; 3.05 biopsies per patient identified with advanced fibrosis). No significant differences were observed if patients were preselected based on the presence of NAFLD (23 biopsies for every 100 patients screened) or abnormal ALT (24 biopsies out of 100 patients screened). The number needed for biopsies to diagnose one patient with advanced fibrosis with these approaches was 2.81 and 2.95, respectively. The use of elevated HOMA-IR for identification of patients allowed to reduce the number of biopsies to 18 for every 100 patients screened, but sensitivity dropped from 76% to 69% (i.e., would result in missing 0.7 patients with advanced fibrosis for every 100 patients screened).
Using AST, AST/ALT ratio, or other ALT cut-off levels to select patients
As the development of fibrosis in NAFLD has been associated with increasing plasma AST and AST/ALT ratio, we also assessed if selecting patients by these would be helpful for targeting screening. However, plasma AST and AST/ALT ratio performed very similarly to plasma ALT, without significant changes in the number needed to biopsy or overall sensitivity (data not shown). Using ALT to select patients with a more conservative cut-off point of > 40 IU/L led to a reduction in the need for biopsies per 100 patients screened with FIB-4 index (i.e., 19 compared to 25 with the cut-off points of 19 and 30 IU/L depending on sex), but also a significant drop in sensitivity (i.e., from 76% to 66%). Similar changes were observed if the higher ALT threshold was used with NAFLD fibrosis score or APRI as the screening tool.
Screening for clinically significant fibrosis
In Table 5, we have provided information regarding the use of these different strategies for the identification of patients with clinically significant fibrosis (n = 49). As expected, these approaches were less sensitive to detecting patients with clinically significant fibrosis but had lower false positive rates. Universal screening using FIB-4 allowed to identify 76% of patients with clinically significant fibrosis but required 3.08 biopsies per patient identified (41 biopsies per 100 patients screened). This could be reduced to ~2 biopsies needed to identify one patient with clinically significant fibrosis by targeting patients with elevated ALT or presence of NAFLD (with sensitivity only minimally reduced to 73%). Using the NFS resulted in overall higher sensitivities, but with higher false positive rates. Similar to what happened with the FIB-4 index, targeting patients with elevated plasma ALT allowed to diminish the number of needed biopsies without significantly affecting the sensitivity. On average, this approach required 2.96 biopsies for every patient identified with clinically significant fibrosis with an overall sensitivity of 94%. Finally, screening with APRI resulted in a similar sensitivity than with the FIB-4 index, but required a lower number of biopsies. Moreover, universal screening with APRI did not result in a significant increase in the number of biopsies needed compared to other selection strategies like elevated ALT or the presence of NAFLD.
Performance of different strategies to identify patients with clinically significant fibrosis (≥ F2) based on FIB-4 index, NFS, and APRI
| Selection strategy | Number of patients selected | Patients with a positive test | Patients identified with clinically significant fibrosis | Number of biopsies per patient identified with clinically significant fibrosis | Patients that would need a biopsy per 100 screened | Sensitivity (%) | False positive rate (%) |
| FIB-4 index ≥ 1.30 | |||||||
| Metabolic approach HOMA-IR ≥ 3 | 129 | 67 | 32 | 2.09 | 24 | 65 | 15 |
| Diabetes approach Patients with T2D | 169 | 87 | 31 | 2.80 | 32 | 63 | 25 |
| Imaging approach NAFLD by 1H-MRS | 183 | 74 | 36 | 2.06 | 27 | 73 | 17 |
| Liver biochemistry approach ALT ≥ 30 IU/L in males and ≥ 19 IU/L in females | 168 | 70 | 36 | 1.94 | 25 | 73 | 15 |
| Universal screening All patients | 275 | 114 | 37 | 3.08 | 41 | 76 | 34 |
| NAFLD fibrosis score ≥ -1.455 | |||||||
| Metabolic approach HOMA-IR ≥ 3 | 129 | 116 | 40 | 2.90 | 42 | 82 | 35 |
| Diabetes approach Patients with T2D | 169 | 151 | 37 | 4.08 | 56 | 76 | 52 |
| Imaging approach NAFLD by 1H-MRS | 183 | 147 | 45 | 3.27 | 54 | 92 | 46 |
| Liver biochemistry approach ALT ≥ 30 IU/L in males and ≥ 19 IU/L in females | 168 | 136 | 46 | 2.96 | 50 | 94 | 41 |
| Universal screening All patients | 275 | 229 | 47 | 4.87 | 85 | 96 | 83 |
| APRI ≥ 0.50 | |||||||
| Metabolic approach HOMA-IR ≥ 3 | 129 | 50 | 33 | 1.67 | 18 | 67 | 8 |
| Diabetes approach Patients with T2D | 169 | 43 | 28 | 1.54 | 16 | 57 | 7 |
| Imaging approach NAFLD by 1H-MRS | 183 | 62 | 35 | 1.77 | 23 | 71 | 12 |
| Liver biochemistry approach ALT ≥ 30 IU/L in males and ≥ 19 IU/L in females | 168 | 65 | 36 | 1.81 | 24 | 73 | 13 |
| Universal screening All patients | 275 | 67 | 36 | 1.86 | 24 | 73 | 14 |
Other sensitivity analyses
We also repeated the analyses limiting the cohort to obese patients (BMI ≥ 30kg/m2), without observing significant differences. Finally, we repeated the analyses, excluding 92 patients that did not have a liver biopsy. These patients did not have NAFLD based on liver 1H-MRS and had normal plasma aminotransferases and therefore were not offered a percutaneous liver biopsy as this would be considered unethical. Because all these patients had a negative
DISCUSSION
The best strategy to identify patients that would benefit from advanced liver fibrosis screening among overweight and obese patients is currently unknown. In the current work, we showed that using non-invasive strategies (i.e., FIB-4 index, NFS, or APRI), universal screening of overweight and obese patients is not justified. If broadly applied, this would lead to a significantly higher number of false positive results, compared to more restrictive strategies. Specifically, targeting screening to only patients with evidence of NAFLD or those with elevated ALT led to the most effective screening strategies, decreasing the need for liver biopsies while maintaining the same sensitivity. Strategies focused on selecting patients based on the presence of insulin resistance by HOMA-IR or the presence of diabetes resulted in lower sensitivities.
Recently, the American Gastroenterological Association (AGA), in collaboration with members of other societies, published a NAFLD/NASH Clinical Care Pathway[8]. In this work, the authors identified three groups of patients at greatest risk of NAFLD/NASH-related fibrosis: 1) patients with T2D, 2) patients with two or more metabolic risk factors, and 3) patients with incidental findings of hepatic steatosis or elevated aminotransferases. The authors recommended that these groups of patients would benefit the most from screening for significant liver fibrosis with noninvasive testing. However, these recommendations were based on experts’ opinions, and no prior study has formally assessed the performance of different patient selection strategies for the screening of liver fibrosis. It is well known, however, that patients can have significant liver disease in the absence of plasma aminotransferase elevations[17]. Therefore, it seems intuitive including other ‘at risk’ groups in the guidelines, such as patients with diabetes and those with metabolic risk factors. However, these and other guidelines[4,8,18] recommend initiating the screening for advanced liver fibrosis with non-invasive tests, which rely heavily on plasma aminotransferase elevations, so patients with normal plasma aminotransferases may still go undiagnosed with these tools. Therefore, adding more ‘at risk’ groups may not provide an advantage over just selecting patients based on elevated plasma aminotransferases. Indeed, our results suggest that an approach that only selects patients based on elevated aminotransferases allows for the maximal sensitivity possible (equal to universal screening) with the least number of patients with positive results requiring further testing. The addition of patients based on other criteria (i.e., HOMA-IR, diabetes, presence of NAFLD) did not increase the detection of patients and only resulted in a higher rate of false positives.
For the purposes of this study, the assumption was that patients with a positive screening would undergo a liver biopsy. However, we are aware that this is not what usually occurs in clinical practice, as patients frequently undergo further testing before getting a biopsy. Regardless, the results from our study are still valid, as it helps to delineate the best selection strategy to target the second step of the diagnostic algorithm. As vibration-controlled transient elastography (VCTE) or Fibroscan® becomes more widely available, it is likely that it will be increasingly used to diagnose advanced fibrosis in NAFLD[19]. Moreover, it has been proposed as a second step in the diagnostic algorithm by recent guidelines[8]. However, because it is still not widely available, especially in developing countries, it was important to establish the most effective way to screen for advanced fibrosis using only simple, cheap, and widely available tools.
Screening by means of APRI, instead of the FIB-4 index, resulted in significant changes in the performance of the different selection strategies. Indeed, universal screening with APRI was as effective as other selection strategies (i.e., presence of NAFLD or elevated ALT), reducing the number of needed biopsies while keeping the sensitivity stable. This suggests that APRI, unlike FIB-4 index, may be used in an ‘untargeted’ fashion on overweight and obese patients without the need to pre-identify specific subgroups. Nevertheless, identifying patients with the presence of NAFLD or elevated ALT did not affect the sensitivity of the approach and, if anything, allowed to reduce the number of biopsies by ~1 per 100 patients screened. NFS allowed to increase the detection of patients with advanced fibrosis (higher sensitivity), but this occurred at the expense of a significantly higher false positive rate. Universal screening of overweight and obese patients with NFS is unlikely to be cost-effective as it leads to an overwhelming number of patients with a positive test. Once again, the selection of patients by elevated ALT was the most effective approach, allowing for the lowest number of biopsies while maintaining sensitivity. Because the NFS is calculated based on BMI and the presence of impaired fasting glucose or diabetes, high false positive rates may be related to the characteristics of our cohort. Patients were selected based on increased BMI (≥ 25 kg/m2) and 91% of patients had prediabetes or diabetes, likely related to referral bias.
Overall and liver-related mortality in patients with NAFLD appears to be related to the fibrosis stage, and it significantly increases once the fibrosis stage is 2 or higher[20]. Therefore, identifying patients with clinically significant fibrosis (stages ≥ 2) is essential to provide appropriate counseling and offer potential treatments. While these approaches were not particularly sensitive to detecting patients with clinically significant fibrosis, using FIB-4 index or APRI in patients with elevated ALT allowed to limit biopsies to 24 or 25, respectively, for every 100 patients screened. Moreover, less than two biopsies were needed (1.94 and 1.81, respectively) to identify one patient with clinically significant fibrosis (i.e., > 50% of patients undergoing a liver biopsy had clinically significant fibrosis).
The study has some limitations, many of which are inherent to the nature of the study. As performing a liver biopsy in patients without NAFLD would be unethical, these patients were assumed to not have significant fibrosis. However, as liver fibrosis progresses, the amount of intrahepatic triglyceride can decrease, which can lead to negative imaging for hepatic steatosis. The other important limitation is that our cohort was likely enriched with patients with metabolic abnormalities due to referral and selection bias. Finally, this is a relatively small sample size. Therefore, confirmation in larger cohorts is important before we can extrapolate and generalize our results.
In summary, the current study suggests that when using non-invasive tests to screen for advanced fibrosis (FIB-4, NFS, or APRI) in overweight and obese patients, targeted screening of patients with elevated ALT provides the most cost-effective approach, reducing the number of needed biopsies while maintaining the sensitivity. Universal screening of these patients resulted in unnecessary false positive results without increasing the number of patients identified with advanced fibrosis. Selecting patients based on insulin resistance or the presence of diabetes did not provide any further advantage compared to elevated ALT alone. If confirmed in larger cohorts of patients, our results provide a simple strategy to identify patients to screen for advanced fibrosis among overweight and obese patients, reducing the number of liver biopsies needed to identify these patients.
DECLARATION
Authors’ contributionsStudy design and funding, patient recruitment and follow-up, data acquisition and interpretation, statistical analysis, and writing, editing and final revision of the manuscript, and taking responsibility for the integrity of the data and the accuracy of the data analysis: Bril F
Data interpretation and revision of draft and final manuscript: Godinez Leiva E
Patient recruitment and follow-up, data acquisition and interpretation, and revision of draft and final manuscript: Lomonaco R
Data interpretation and revision of draft and final manuscript: Shrestha S
Data acquisition and interpretation, and revision of draft and final manuscript: Kalavalapalli S
Data interpretation and revision of draft and final manuscript: Gray M
Funding, patient recruitment and follow-up, data acquisition and interpretation, and revision of the draft and final manuscript: Cusi K
Availability of data and materialsData available upon request to corresponding authors.
Financial support and sponsorshipEarly-Career Research Grant from The Obesity Society (F.B.); UF Clinical and Translational Science Institute Pilot Project Award (F.B.); and American Diabetes Association (1-08-CR-08 [K. C.]). Research reported in this publication was supported by the UF Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Conflicts of interestNothing to disclose in relation to this manuscript.
Ethical approval and consent to participateThe study was approved by the institutional review boards at the University of Florida (UF 53-2012; 201600202; 201300679) and University of Texas Health Science Center at San Antonio (HSC20070654), and written informed consent was obtained from each patient prior to their participation.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.
2. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11-20.
3. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793-801.
4. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57.
5. Bril F, McPhaul MJ, Caulfield MP, et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care 2020;43:290-7.
6. Mózes FE, Lee JA, Selvaraj EA, et al. LITMUS Investigators. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-19.
7. European Association for the Study of the Liver (EASL)., European Association for the Study of Diabetes (EASD)., European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402.
8. Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657-69.
9. Bril F, McPhaul MJ, Kalavalapalli S, et al. Intact fasting insulin identifies nonalcoholic fatty liver disease in patients without diabetes. J Clin Endocrinol Metab 2021;106:e4360-71.
10. Bril F, Portillo-Sanchez P, Liu IC, et al. Clinical and histologic characterization of nonalcoholic steatohepatitis in African American patients. Diabetes Care 2018;41:187-92.
11. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305-15.
12. Bril F, Ortiz-Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015;35:2139-46.
13. Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016;59:1121-40.
14. Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344-53.
15. Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21.
16. Godinez-Leiva E, Bril F. Nonalcoholic Fatty Liver Disease (NAFLD) for primary care providers: beyond the liver. Curr Hypertens Rev 2021;17:94-111.
17. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231-8.
18. Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care 2022;45:S46-59.
19. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399-406.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Bril F, Godinez Leiva E, Lomonaco R, Shrestha S, Kalavalapalli S, Gray M, Cusi K. Assessing strategies to target screening for advanced liver fibrosis among overweight and obese patients. Metab Target Organ Damage 2022;2:11. http://dx.doi.org/10.20517/mtod.2022.08
AMA Style
Bril F, Godinez Leiva E, Lomonaco R, Shrestha S, Kalavalapalli S, Gray M, Cusi K. Assessing strategies to target screening for advanced liver fibrosis among overweight and obese patients. Metabolism and Target Organ Damage. 2022; 2(3): 11. http://dx.doi.org/10.20517/mtod.2022.08
Chicago/Turabian Style
Bril, Fernando, Eddison Godinez Leiva, Romina Lomonaco, Sulav Shrestha, Srilaxmi Kalavalapalli, Meagan Gray, Kenneth Cusi. 2022. "Assessing strategies to target screening for advanced liver fibrosis among overweight and obese patients" Metabolism and Target Organ Damage. 2, no.3: 11. http://dx.doi.org/10.20517/mtod.2022.08
ACS Style
Bril, F.; Godinez Leiva E.; Lomonaco R.; Shrestha S.; Kalavalapalli S.; Gray M.; Cusi K. Assessing strategies to target screening for advanced liver fibrosis among overweight and obese patients. Metab Target Organ Damage. 2022, 2, 11. http://dx.doi.org/10.20517/mtod.2022.08
About This Article
Copyright
Data & Comments
Data
 Cite This Article 26 clicks
Cite This Article 26 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.