Effect of cofactors on NAFLD/NASH and MAFLD - a paradigm illustrating the pathomechanics of organ dysfunction
Abstract
Primary nonalcoholic fatty liver disease (NAFLD) is bi-directionally associated with the metabolic syndrome and its constitutive features (“factors”: impaired glucose disposal, visceral obesity, arterial hypertension, and dyslipidemia). Secondary NAFLD occurs due to endocrinologic disturbances or other cofactors. This nosography tends to be outdated by the novel definition of metabolic associated fatty liver disease (MAFLD). Irrespective of nomenclature, this condition exhibits a remarkable pathogenic heterogeneity with unpredictable clinical outcomes which are heavily influenced by liver histology changes. Genetics and epigenetics, lifestyle habits [including diet and physical (in)activity] and immunity/infection appear to be major cofactors that modulate NAFLD/MAFLD outcomes, including organ dysfunction owing to liver cirrhosis and hepatocellular carcinoma, type 2 diabetes, chronic kidney disease, heart failure, and sarcopenia. The identification of cofactors for organ dysfunction that may help understand disease heterogeneity and reliably support inherently personalized medicine approaches is a research priority, thus paving the way for innovative treatment strategies.
Keywords
DEFINITIONS AND BURDEN
Nonalcoholic fatty liver disease (NAFLD) is an umbrella definition encompassing the clinico-pathological spectrum of disorders spanning from simple steatosis to nonalcoholic steatohepatitis (NASH), with or without fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[1,2]. This implies that NAFLD defines a gamut of conditions mimicking alcohol-related liver disease but are seen in patients without alcohol use disorder[3]. In principle, in addition to alcohol, other competing causes of liver disease should be ruled out in the NAFLD field, notably including HCV infection and thyroid disorders, although the extent to which alternative etiologies need to be excluded remains poorly defined[4]. Moreover, the rationale for distinguishing alcohol-related liver disease from NAFLD may sometimes appear uncertain[5,6].
Clinically and epidemiologically, NAFLD is important given that it exacts a heavy toll in terms of patient quality of life[7] and, owing to direct and indirect costs, accounts for substantial healthcare expenditures[8-10].
CLASSIFICATION
Based on its pathogenic framework, NAFLD may be categorized as either primary or secondary disease. Primary NAFLD exhibits a mutual and bi-directional association with the metabolic syndrome and its individual components: impaired glucose disposal, visceral obesity, atherogenic dyslipidemia, and arterial hypertension[11]. These “factors” tend to cluster, such that the appearance of each predicts the future development of others[12]. However, there are also several secondary NAFLD forms[13]. These secondary NAFLD forms may, in their turn, be classified as illustrated in Table 1[13-26].
Secondary NAFLD forms
| Etiology | Comment | Authors |
| Viral-HCV Viral-HIV | There are two different types of steatosis owing to HCV infection. HCV genotype 3 is directly steatogenic and has steatosis which is more common and consistent, whereas HCV genotypes other than genotype 3 exhibit lower prevalence and severity of steatosis, which is associated with the host’s metabolic determinants HCV steatosis occurs in the setting of a complex pattern of metabolic alterations named “hepatitis C-associated dysmetabolic syndrome” (HCADS) also featuring hepatic steatosis; visceral fat hypertrophy; acquired, reversible hypocholesterolemia; and insulin resistance Strictly speaking, HCV-related steatosis cannot be classified as NAFLD and should best be named “MAFLD” HIV infection is strongly associated with steatosis. Formerly defined as “VAFLD” (virus-associated fatty liver disease), this entity should presently best be renamed “MAFLD” | Adinolfi et al.[14] Lonardo et al.[15] Polyzos et al.[16] Guaraldi et al.[17] Liu et al.[18] |
| Nutritional/intestinal-related causes | A variety of medico-surgical conditions, including acute weight loss (bariatric surgery and fasting), malnutrition, total parenteral nutrition, short bowel syndrome, intestinal failure, small intestinal bacterial overgrowth, microbiome changes, coeliac disease, and pancreatectomy, may lead to secondary NAFLD forms, some of which are highly progressive to cirrhosis | Liebe et al.[13] Angulo et al.[19] |
| Endocrine NAFLD/NASH | Polycystic ovary syndrome (PCOS), hypothyroidism, hypogonadism, and GH deficiency may be conceptualized as a naturally occurring disease model of NAFLD, which have specific pathomechanisms and are potentially reversible with specific treatment | Lonardo et al.[20] |
| Associated with pregnancy | Acute fatty liver of pregnancy | Azzaroli et al.[21] |
| Associated with metals and synthetic chemicals | Metals (such as lead) have been implicated in more fibrotic NAFLD forms in the NHANES population Environmental chemicals of industrial, agricultural, residential, and pharmaceutical origin can disrupt endocrine-metabolic pathways leading to secondary NAFLD forms | Reja et al.[22] Heindel et al.[23] Cano et al.[24] |
| Genetic disorders of metabolism | A variety of common and rare inherited metabolic disorders such as hemochromatosis, alpha-1 antitrypsin deficiency, Wilson’s disease, congenital lipodystrophy, glycogen storage diseases, hereditary fructose intolerance, urea cycle disorders, and citrullinaemia type 2 are associated with secondary NAFLD forms or worsen primary NAFLD | Liebe et al.[13] Angulo et al.[19] |
| Drug-related | Many drugs can be steatogenic, including antiretrovirals, tamoxifen, corticosteroids, tetracyclines, valproic acid, amphetamines, and acetylsalicylic acid. However, drug-induced liver injury (DILI) is a definite disease entity other than NAFLD Additionally, NAFLD patients may be at high risk of developing DILI, demonstrating that these are two different disease entities with some shared pathogenic aspects | Lammert et al.[25] Tarantino et al.[26] |
Although the topic remains open for discussion, notions reported in Table 1 suggest that the most common secondary NAFLD forms occur in the setting of specific endocrine derangements and inherited metabolic disorders.
NAFLD vs. MAFLD - LIMITATIONS OF LIVER BIOPSY
The proposal to rename NAFLD to metabolic-associated fatty liver disease (MAFLD)[27,28], which has met a universally favorable reception[16,29], appears to be a logical attempt to overcome the two principal limitations and inconsistencies inherent in the NAFLD/NASH definition (discussed below) while emphasizing the association of hepatic fatty changes with the metabolic syndrome and its components[30]. The main drawbacks of the NAFLD nosography include: (a) liver biopsy; and (b) exclusion of alcohol consumption. (a) The practice of liver biopsy in NAFLD arena must be considered with a prudent and balanced view. On the one hand, NASH is a clinico-pathological disease entity that, by its very definition, requests histological documentation[31]. On the other hand, liver biopsy is invasive, not painless, nor devoid of stress for the patient; it may carry risky complications such as bleeding and perforation of hollow organs; and may even be (rarely) mortal[32-34]. Moreover, liver histology changes may be patchily distributed through the hepatic parenchyma opening to disease misclassification from sampling error[35,36], and it is fibrosis (which can also be assessed non-invasively), not NASH, that dictates the prognosis in NAFLD[37]. Finally, it is uncertain whether - for clinical practice purposes - we do have to perform an invasive and potentially risky procedure without prescribing any approved drugs or biological treatments for NASH or related liver diseases to our patients[34,38]. All the above perplexities may be overcome by the less committal diagnosis of MAFLD, which does not request liver biopsy[39]. (b) There are no objective and reliable biomarkers of alcohol consumption to define whether a given liver disease is “alcoholic” or “nonalcoholic”[40]. Unless stated otherwise, it is difficult to objectively determine the number of alcoholic units consumed or, alternatively, the duration of alcohol abstinence. At the same time, the rationale for separating alcohol-related from nonalcoholic liver disease has also been deemed to be questionable based on histological and pathogenic grounds[41,42]. Collectively, those arguments summarized above under Points (a) and (b) further reinforce the rationale for transitioning from NAFLD to MAFLD.
NAFLD PATHOGENIC HETEROGENEITY AND HISTOLOGICAL BASES OF CLINICAL VARIABILITY
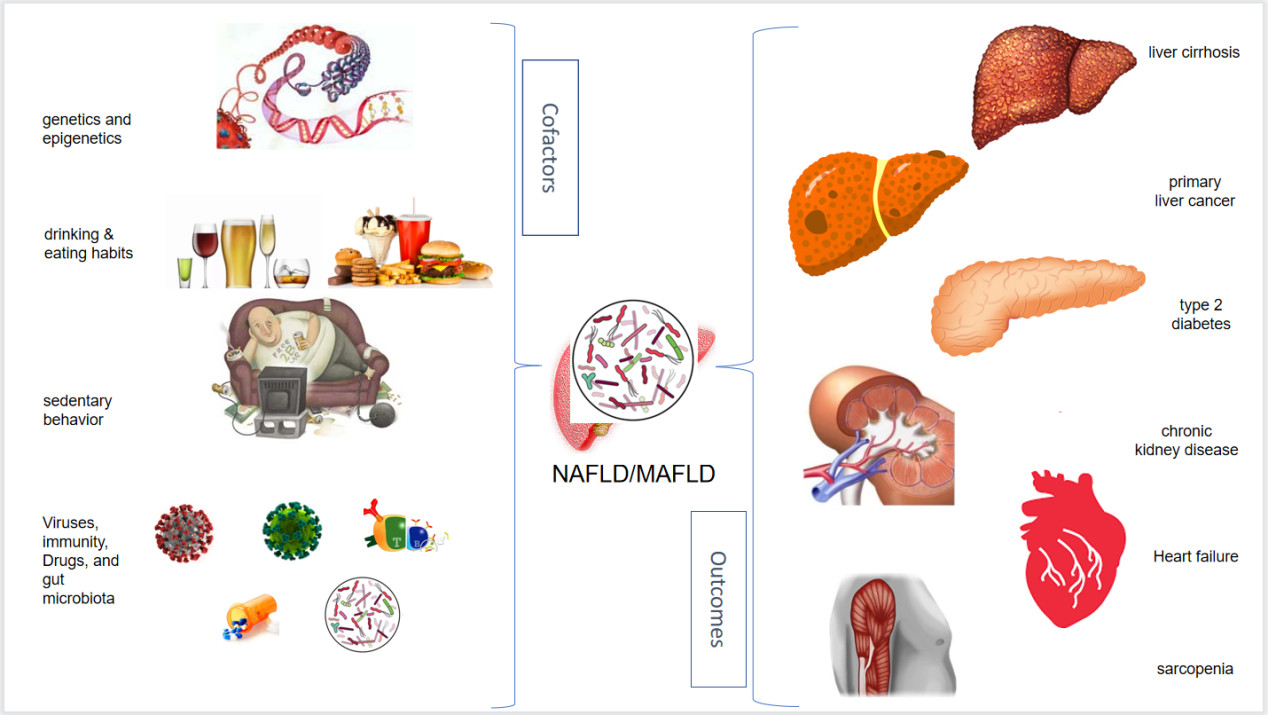
NAFLD exhibits some prominent features that uniquely characterize its pathophysiological and clinical profile. First, it is a systemic disorder whose manifestations reach far beyond the liver[43,44]. Second, it has a remarkable pathogenic heterogeneity and runs an unpredictable course in the individual patient[45]. Third, it has a distinct sexual dimorphism[46,47]. Ideally, it would be tempting to speculate that it is pathogenic heterogeneity, including the impact of sex and reproductive status, that will eventually dictate a natural course in any given patient. Although this notion is reasonable, we are still far from having clear evidence for this conclusion. What is certain is that the course of NAFLD exhibits a remarkable variety of target organ dysfunction[48]. This spans from the liver (cirrhosis and HCC) (2), pancreatic beta-cell (diabetes)[49], the kidneys (chronic kidney disease)[50], the skeletal (sarcopenia), cardiac muscles (heart failure)[51,52], and the lungs (impaired function)[53,54] to the development of cancer in a variety of organs[55,56]. What then determines such an impressively diverse clinical course in the individual subject?
In 2018, Vilar Gomez et al. published a breakthrough study in the area, identifying liver histology as a determinant of hepatic versus extrahepatic disease manifestations. With a 5.5-year follow-up on a cohort of approximately 460 biopsy-proven NAFLD patients, they found that cirrhosis was associated with predominantly liver-related events, while bridging fibrosis was linked to the development of predominantly non-hepatic cancers and vascular events[57]. Although it would be unimaginable for all patients with NAFLD to undergo a liver biopsy, it is anticipated that either non-invasive biomarkers of fibrosis or imaging techniques quantifying fibrosis may serve as a substitution for liver biopsy in determining the course of disease[58,59]. Again, although perfectly plausible, this hypothesis remains to be tested in further prospective studies.
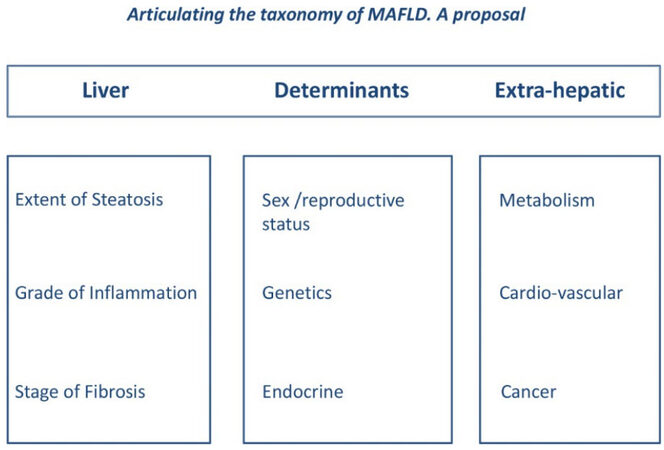
The selection of more homogenous patient populations with more predictable disease outcomes, and presumably higher treatment response rates, represents a research priority due to the disappointing results of many NASH trials[60]. While, in the future, a precise metabolic identity card may best characterize the individual NAFLD patient[61], this tool is not yet available in clinical practice. A feasible strategy for this goal could be identifying similar phenotypic subgroups. Given the systemic nature of NAFLD, a simple classification system should include liver, pathogenic determinants, and extrahepatic (LDE) features, as illustrated in Figure 1.
Figure 1. The LDE system (reprinted from[4]). The LDE system, which may be applied to both NAFLD and MAFLD, exhibits a basic syntax including a prefix (“L” for liver), a pathogenic core (“D” for determinants), and a suffix (“E” for extrahepatic). Liver (L): Information regarding liver health, which may also be obtained non-invasively other than histologically. Determinants (D): Information including sex and reproductive status, genetic determinants, and (minimal) endocrine assessment. Extrahepatic (E): Data on extrahepatic manifestations of disease. For example, illustrating this proposed classification, patient Mr. Max Green might be declared to have MAFLD/NAFLD (L, steatosis mild, inflammation absent, and fibrosis absent; D, hypothyroid, no SNP identified, and associated with full-blown MetS; and E, arterial hypertension, medio-intimal carotid thickening, and previous colon cancer).
NAFLD COFACTORS
There is no unified definition of “cofactors” in the NAFLD/MAFLD field, although this term was extensively evaluated and studied in the HCV arena in the past[62]. In this perspective, we define “cofactors”, such as clinically relevant disease modifiers, as “cofactors” that interact with “metabolic factors” in the field of metabolic syndrome.
Interestingly, these cofactors may have diagnostic implications (e.g., genetics), and some are modifiable (e.g., lifestyle habits and infection). These cofactors are innumerable, and the current perspective does not aim to be exhaustive on the cofactor spectrum. Instead, some of the best-characterized examples of NAFLD cofactors are discussed below. Emphasis is given to those that have been better characterized, are more extensively evaluated, or appear to be more promising.
As illustrated in our Graphical Abstract, the current perspective has five sections: (1) genetics and epigenetics; (2) drinking and eating habits; (3) sedentary behavior; (4) immunity and drugs; and (5) viral infections.
Genetics and epigenetics
Studies demonstrating that first-degree relatives of NAFLD patients exhibit a much higher risk of the disease compared to the general population support the notion that genetics and epigenetics play a key role in the development of NAFLD[63]. Indeed, genome-wide association studies have identified numerous genetic polymorphisms involved in NAFLD development and progression, e.g., patatin-like phospholipase domain-containing protein 3 (PNPLA3), membrane-bound O-acyltransferase domain containing 7 (MBOAT7), transmembrane 6 superfamily member 2 (TM6SF2), glucokinase regulator (GCKR), and others[64,65]. However, whether “metabolic NAFLD” (i.e., MAFLD) and “genetic NAFLD” follow the same natural course remains unproven[66,67]. PNPLA3 variant rs739409: C > G on chromosome 22 is the single most replicated variant in liver diseases and was first identified in 2008 in association with NAFLD[68]. It has now been firmly established as a gene modifier of hepatic steatosis and a risk factor for liver disease progression[69]. It is a non-synonymous single nucleotide mutation altering a highly conserved amino acid isoleucine to methionine at residue 148. PNPLA3 encodes for adiponutrin, a transmembrane protein that has lipogenic transacetylase and triglyceride hydrolase activities. It is suspected that I148M promotes hepatic intracellular lipid accumulation by reducing the breakdown of triglycerides stored in the lipid droplets[70]. A non-synonymous single nucleotide variant in the TM6SF2 (rs58542926: C > T (E167K) on chromosome 19 is associated with hepatic triglyceride content and is an independent risk factor for liver fibrosis and HCC[71]. Recent studies demonstrate that TM6SF2 acts in the smooth endoplasmic reticulum to promote bulk lipidation of apolipoprotein B-containing lipoproteins, thus preventing fatty liver disease[72]. MBOAT7 encodes an enzyme with lysophosphatidylinositol acyltransferase activity, and its variant, rs641738 C > T, is associated with NAFLD[73] and fibrosis in patients with a BMI < 35 independent of lobular inflammation[74]. Importantly, in animal models, its loss of function is sufficient to promote NAFLD progression[75]. Glucokinase regulator (GCKR) encodes glucokinase regulatory protein (GKRP), a hepatocyte-specific inhibitor of the glucose-metabolizing enzyme glucokinase, a primary glucose sensor[76].
Epigenetic mechanisms, comprising histone methylation, abnormal DNA methylation, and circulating miRNA profiles, all interact with inherited risk factors to determine individual susceptibility to NAFLD and, compared to genetic mechanisms, are affected by the patient’s lifestyle changes[64,77,78]. The finding that adaptions to maternal obesity in early life increase the susceptibility to developing NAFLD and its complications in offspring[79] is an excellent example of the role of epigenetic factors in NAFLD pathobiology. Hagström et al., in their population-based study recruiting 125 biopsy-proven cases compared to 717 controls, consistently found that maternal BMI early in pregnancy was an independent risk factor for the diagnosis and severity of NAFLD in their offspring (OR in offspring to obese mothers: 3.26, CI 1.72-6.19, for any NAFLD and 3.67, CI 1.61-8.38, for fibrotic NAFLD)[80]. This study indirectly suggests that educational campaigns aimed at improving diet and encouraging physical exercise would reduce the risk of obesity-related conditions in mothers and their offspring and should be conducted among obese women of fertile age[80]. Interestingly, evaluation of liver transcriptome profiles in rats has shown that maternal obesity programs sex-dependent changes in offspring hepatic gene expression leading to more severe insulin resistance and NAFLD among male offspring than female counterparts[81]. Moreover, by comparing germ-free mice colonized with stool microbes from two-week-old infants born to either obese or normal-weight mothers, Soderborg et al. demonstrated that altered gut microbiome composition (i.e., dysbiosis) results in increased hepatic inflammatory responses and triggers NAFLD and excess weight gain in germ-free mice colonized with stool microbes from two-week-old infants born to obese mothers[82].
Together, genetic and epigenetic cofactors participate in NAFLD development and progression and carry translational implications, which can be exploited to implement personalized medicine approaches[64,83]. These include programs for targeted screening and surveillance of complications, prediction of the individual response to pharmacological therapies, and opportunities for using miRNAs for treating liver disease and utilizing the gene variant as the therapeutic target[78,84]. Lifestyle habits predisposing to the development and progression of NAFLD represent a holistic scenario including sedentary behavior and unhealthy dietary patterns, which are discussed below under Points 2 and 3.
Eating habits
If the Mediterranean diet (Med-diet), featuring homemade, unprocessed plant-based foods as well as fish and poultry in low to moderate amounts, is deemed to protect from NAFLD and NASH, the growing global consumption of ultra-processed hypercaloric foods enriched in simple sugars and hydrogenated fats is deemed to facilitate the metabolic syndrome, steatosis, and its histological progression[85]. These notions have tremendous clinical potential in as much as they indicate what NAFLD/NASH/MAFLD patients should be suggested to eat[86]. For example, a meta-analysis and meta-regression analysis of six randomized controlled trials found that - compared to the control diet - Med-Diet was associated with significant reductions of fatty liver index (FLI) and homeostasis model assessment of insulin resistance (HOMA-IR), suggesting that Med-Diet is a beneficial pharmaco-nutritional therapy in NAFLD[87].
Additionally, recent studies refute the classic notion that moderate alcohol consumption might be beneficial in NAFLD; thus, alcohol should best be avoided per guideline recommendations (reviewed in[87]). Concerns about the potential linear dose-response on the pro-fibrogenic and carcinogenic effect of alcohol[88-92] fully support the notion that alcohol is a cofactor that potentially causes target organ dysfunction[93-95]. Dietary habits are inextricably connected with physical activity patterns.
Physical (in) activity
Studies have shown that NAFLD, physical inactivity and depressive symptoms form a dangerous pathogenic triangle[4,96,97]. Weinstein et al. provided proof-of-concept of this notion by analyzing the Rancho Bernardo Study of Healthy Aging. Overall, 589 individuals were included in the analyses. Data show that individuals with NAFLD have high levels of physical inactivity, particularly those with depressive symptoms[98]. Of concern, a low level of physical activity, in turn, is associated both with an increased NAFLD prevalence and with unfavorable cardio-metabolic and hepatic outcomes of NAFLD[99,100]. Thus, increasing physical activity remains an undisputed mainstay for preventing and managing NAFLD and related organ dysfunction. Additionally, lifestyle habits are known to be associated with immunity patterns.
Infection, immunity, and microbiota
While NAFLD is deemed to predispose to a variety of infections, including bacterial[101], the impact of infection on NAFLD course has mostly focused on viral infections. Probably the earliest and best-characterized examples include viral hepatitis C and B[15,102,103]. Additionally, many data address the deleterious interaction of NAFLD with HIV infection[104,105]. More recently, researchers have focused on SARS-CoV-2 infection[106-108]. Collectively, data suggest that viral infections are strongly associated with NAFLD outcomes, implying their role as disease cofactors. Immune dysfunction is a vast and under-appreciated aspect that likely plays a wide range of pathogenic roles spanning from NAFLD pathobiology and progression[109,110] and the interaction of NAFLD with autoimmune (liver) disorders[111-113] to drug-induced liver injury occurring in NAFLD individuals[114,115]. Whether and which tests exploring immune dysfunction in NAFLD should be used to better characterize NAFLD phenotypes remains to be defined.
Gut microbes comprise bacteria, fungi, viruses, archaea, and protozoa. The bacterial microbiome in healthy humans is dominated by beneficial bacterial phyla such as Bacteroides and Firmicutes, and a smaller proportion consists of Proteobacteria, Actinobacteria, and Verrucomicrobia[116]. The gut bacterial microbiome in patients with liver disease is characterized by dysbiosis with an increase in harmful and a decrease in beneficial bacteria, and this abnormality worsens with increased disease severity and is also associated with liver and patient-related outcomes[117].
Although the exact role of gut microbiome in the pathogenesis of NAFLD remains unclear, there is a characteristic microbiome profile observed in NAFLD patients, with lower diversity and increased proportion of Coprococcus, Ruminococcus, Proteobacteria, and Enterobacteriaceae spp. NASH patients with advanced fibrosis, compared to those with or without early-stage fibrosis, had a higher proportion of Proteobacteria and E. coli, with a lower proportion of Firmicutes, especially F. prausnitzii[118,119]. Conflicting with their pathogenic and clinical significance, data regarding the qualitative and quantitative composition of intestinal microbiota have not yet entered the clinical arena.
CONCLUSION
From a conceptual perspective, the NAFLD/NASH nosography continues to offer the advantages of precisely ruling out competing causes of liver disease (e.g., alcohol, viral infection, and others) and accurately describing liver histology changes. However, these do not necessarily need to be ruled out and reported in MAFLD diagnosis. MAFLD, on the other hand, probably offers the advantage of more accurately identifying the risk of target organ dysfunction, namely, progressive liver disease[120], diabetes and chronic kidney disease[121], atherosclerosis[122], more severely impaired lung function[123], colon cancer[124], both intrahepatic and extrahepatic events[125], and mortality[126], although the last outcome is controversial[127].
With this evolving scenario, the identification of cofactors for organ dysfunction[128], which may contribute to explaining disease heterogeneity and consistently support inherently personalized medicine approaches, has been suggested as a possible solution to overcome the issue of non-responders to conventional therapeutic approaches in metabolic disorders and failures of NASH therapeutic trials[9,129,130]. To this end, an ever-increasing awareness of the type, number, and significance of NAFLD/NASH and MAFLD cofactors is a research priority, which opens the way to innovative pathogenic treatment strategies in this field.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of this Perspective and wrote the first draft of the manuscript: Lonardo A
Critical revision of content and language: Kharbanda KK, Osna N, Singal AK
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported in part by the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review grants, BX004053 (KKK) and National Institute of Health grants, R01AA026723 (KKK) & R01AA027189 (NO).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Association for the Study of the Liver (EASL)., European Association for the Study of Diabetes (EASD)., European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402.
2. Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis 2017;49:471-83.
3. Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology 1988;95:1056-62.
4. Lonardo A, Ballestri S. Perspectives of nonalcoholic fatty liver disease research: a personal point of view. Explor Med 2020;1:85-107.
5. Craciun A, Lackner C, Cortez-Pinto H. Nonalcoholic fatty liver disease versus alcohol-related liver disease: is it really so different? Curr Pharm Des 2020;26:1093-109.
6. Singal AK. Similarities and differences between non-alcoholic fatty liver disease (NAFLD) & alcohol-associated liver disease (ALD). Transl Gastroenterol Hepatol 2021;6:1.
7. Younossi ZM, Yilmaz Y, Yu ML, et al. Global NASH Council. Clinical and patient-reported outcomes from patients with nonalcoholic fatty liver disease across the world: data from the global Non-Alcoholic Steatohepatitis (NASH)/ Non-Alcoholic Fatty Liver Disease (NAFLD) registry. Clin Gastroenterol Hepatol 2021:S1542-3565(21)01183.
8. Sayiner M, Arshad T, Golabi P, Paik J, Farhat F, Younossi ZM. Extrahepatic manifestations and healthcare expenditures of non-alcoholic fatty liver disease in the Medicare population. Hepatol Int 2020;14:556-66.
9. Gordon S, Fraysse J, Li S, Ozbay AB, Wong RJ. Disease severity is associated with higher healthcare utilization in nonalcoholic steatohepatitis medicare patients. Am J Gastroenterol 2020;115:562-74.
10. Wong RJ, Kachru N, Martinez DJ, Moynihan M, Ozbay AB, Gordon SC. Real-world comorbidity burden, health care utilization, and costs of nonalcoholic steatohepatitis patients with advanced liver diseases. J Clin Gastroenterol 2021;55:891-902.
11. Lonardo A, Leoni S, Alswat KA, Fouad Y. History of nonalcoholic fatty liver disease. Int J Mol Sci 2020;21:5888.
12. Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 2015;47:181-90.
13. Liebe R, Esposito I, Bock HH, et al. Diagnosis and management of secondary causes of steatohepatitis. J Hepatol 2021;74:1455-71.
14. Adinolfi LE, Rinaldi L, Guerrera B, et al. NAFLD and NASH in HCV infection: prevalence and significance in hepatic and extrahepatic manifestations. Int J Mol Sci 2016;17:803.
15. Lonardo A, Adinolfi LE, Restivo L, et al. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol 2014;20:7089-103.
16. Polyzos SA, Kang ES, Tsochatzis EA, et al. Commentary: nonalcoholic or metabolic dysfunction-associated fatty liver disease? Metabolism 2020;113:154413.
17. Guaraldi G, Lonardo A, Ballestri S, et al. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus-associated fatty liver disease. Arch Med Res 2011;42:690-7.
18. Liu D, Shen Y, Zhang R, et al. Prevalence and risk factors of metabolic associated fatty liver disease among people living with HIV in China. J Gastroenterol Hepatol 2021;36:1670-8.
20. Lonardo A, Mantovani A, Lugari S, Targher G. NAFLD in some common endocrine diseases: prevalence, pathophysiology, and principles of diagnosis and management. Int J Mol Sci 2019;20:2841.
21. Azzaroli F, Mazzella G, Marchesini G, Brodosi L, Petroni ML. Fatty liver in pregnancy: a narrative review of two distinct conditions. Expert Rev Gastroenterol Hepatol 2020;14:127-35.
22. Reja D, Makar M, Visaria A, Karanfilian B, Rustgi V. Blood lead level is associated with advanced liver fibrosis in patients with non-alcoholic fatty liver disease: a nationwide survey (NHANES 2011-2016). Ann Hepatol 2020;19:404-10.
23. Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 2017;68:3-33.
24. Cano R, Pérez JL, Dávila LA, et al. Role of Endocrine-Disrupting chemicals in the pathogenesis of non-alcoholic fatty liver disease: a comprehensive review. Int J Mol Sci 2021;22:4807.
25. Lammert C, Imler T, Teal E, Chalasani N. Patients with chronic liver disease suggestive of nonalcoholic fatty liver disease may be at higher risk for drug-induced liver injury. Clin Gastroenterol Hepatol 2019;17:2814-5.
26. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res 2007;37:410-5.
27. Eslam M, Sanyal AJ, George J. International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999-2014.e1.
28. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202-9.
29. Méndez-sánchez N, Bugianesi E, Gish RG, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 2022;7:388-90.
30. Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res 2016;46:1074-87.
31. Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc 2015;90:1233-46.
32. Hilzenrat N, Yesovitch R, Shrier I, Stavrakis M, Deschênes M. The effect of information level and coping style on pain and anxiety in needle liver biopsy. Can J Gastroenterol 2006;20:597-600.
33. Midia M, Odedra D, Shuster A, Midia R, Muir J. Predictors of bleeding complications following percutaneous image-guided liver biopsy: a scoping review. Diagn Interv Radiol 2019;25:71-80.
34. Neuberger J, Patel J, Caldwell H, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020;69:1382-403.
35. Ratziu V, Charlotte F, Heurtier A, et al. LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898-906.
36. Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20:15539-48.
37. Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265-73.
38. Harvey BE. NASH: regulatory considerations for clinical drug development and U.S. FDA approval. Acta Pharmacol Sin 2022;43:1210-4.
39. Segura-Azuara NLÁ, Varela-Chinchilla CD, Trinidad-Calderón PA. MAFLD/NAFLD biopsy-free scoring systems for hepatic steatosis, NASH, and fibrosis diagnosis. Front Med (Lausanne) 2021;8:774079.
40. Arnts J, Vanlerberghe BTK, Roozen S, et al. Diagnostic accuracy of biomarkers of alcohol use in patients with liver disease: a systematic review. Alcohol Clin Exp Res 2021;45:25-37.
41. Greuter T, Malhi H, Gores GJ, Shah VH. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis. JCI Insight 2017;2:95354.
42. Idalsoaga F, Kulkarni AV, Mousa OY, Arrese M, Arab JP. Non-alcoholic fatty liver disease and alcohol-related liver disease: two intertwined entities. Front Med (Lausanne) 2020;7:448.
43. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis 2015;35:236-49.
44. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol 2021;6:578-88.
46. Lonardo A, Suzuki A. Sexual dimorphism of NAFLD in adults. Focus on clinical aspects and implications for practice and translational research. J Clin Med 2020;9:1278.
47. Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021;19:61-71.e15.
48. Ballestri S, Mantovani A, Nascimbeni F, Lugari S, Lonardo A. Extra-hepatic manifestations and complications of nonalcoholic fatty liver disease. Future Med Chem 2019;11:2171-92.
49. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 2021;70:962-9.
50. Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut 2022;71:156-62.
51. Arrese M, Cabello-verrugio C, Arab JP, et al. Sarcopenia in the setting of nonalcoholic fatty liver. Metab Target Organ Damage 2022;2:2.
52. Mantovani A, Byrne CD, Benfari G, Bonapace S, Simon TG, Targher G. Risk of heart failure in patients with nonalcoholic fatty liver disease: JACC review topic of the week. J Am Coll Cardiol 2022;79:180-91.
53. Mantovani A, Lonardo A, Vinco G, et al. Association between non-alcoholic fatty liver disease and decreased lung function in adults: a systematic review and meta-analysis. Diabetes Metab 2019;45:536-44.
54. Lonardo A, Beghe B, Fabbri LM. Chronic obstructive pulmonary disease (COPD) and metabolic fatty liver syndromes: a dangerous but neglected liaison. Metab Target Organ Damage 2022;2:6.
55. Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022;71:778-88.
57. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 2018;155:443-457.e17.
58. Selvaraj EA, Mózes FE, Jayaswal ANA, et al. LITMUS Investigators. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. J Hepatol 2021;75:770-85.
59. Mózes FE, Lee JA, Selvaraj EA, et al. LITMUS Investigators. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-19.
60. Ratziu V, Friedman SL. Why do so many NASH trials fail? Gastroenterology 2020:S0016-5085(20)30680.
61. Lonardo A. Metabolomic signature: one step forward in the process of obtaining NAFLD patients’ metabolic identity card. Am J Clin Nutr 2022;115:603-5.
62. Persico M, Bruno S, Costantino A, Mazza M, Almasio PL. The impact of antiviral therapy and the influence of metabolic cofactors on the outcome of chronic HCV infection. Int J Hepatol 2011;2011:314301.
63. Long MT, Gurary EB, Massaro JM, et al. Parental non-alcoholic fatty liver disease increases risk of non-alcoholic fatty liver disease in offspring. Liver Int 2019;39:740-7.
64. Botello-Manilla AE, Chávez-Tapia NC, Uribe M, Nuño-Lámbarri N. Genetics and epigenetics purpose in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol 2020;14:733-48.
65. Arrese M, Arab JP, Barrera F, Kaufmann B, Valenti L, Feldstein AE. Insights into nonalcoholic fatty-liver disease heterogeneity. Semin Liver Dis 2021;41:421-34.
66. Petäjä EM, Yki-Järvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD-A systematic review. Int J Mol Sci 2016;17:633.
67. Lonardo A, Ballestri S, Targher G. Not all forms of NAFLD were created equal. Do metabolic syndrome-related NAFLD and PNPLA3-related NAFLD exert a variable impact on the risk of early carotid atherosclerosis? Atherosclerosis 2017;257:253-5.
68. Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461-5.
69. Yuan L, Terrrault NA. PNPLA3 and nonalcoholic fatty liver disease: towards personalized medicine for fatty liver. Hepatobiliary Surg Nutr 2020;9:353-6.
70. Pirazzi C, Adiels M, Burza MA, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol 2012;57:1276-82.
71. Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2014;46:352-6.
72. Luo F, Smagris E, Martin SA, et al. Hepatic TM6SF2 is required for lipidation of VLDL in a Pre-Golgi compartment in mice and rats. Cell Mol Gastroenterol Hepatol 2022;13:879-99.
73. Mancina RM, Dongiovanni P, Petta S, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of european descent. Gastroenterology 2016;150:1219-1230.e6.
74. Thangapandi VR, Knittelfelder O, Brosch M, et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut 2021;70:940-50.
75. Helsley RN, Varadharajan V, Brown AL, et al. Obesity-linked suppression of membrane-bound O-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. Elife 2019;8:e49882.
76. Schaftingen E. A protein from rat liver confers to glucokinase the property of being antagonistically regulated by fructose 6-phosphate and fructose 1-phosphate. Eur J Biochem 1989;179:179-84.
77. Campo JA, Gallego-Durán R, Gallego P, Grande L. Genetic and epigenetic regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int J Mol Sci 2018;19:911.
78. Rodríguez-Sanabria JS, Escutia-Gutiérrez R, Rosas-Campos R, Armendáriz-Borunda JS, Sandoval-Rodríguez A. An update in epigenetics in metabolic-associated fatty liver disease. Front Med (Lausanne) 2021;8:770504.
79. Thompson MD. Developmental programming of NAFLD by parental obesity. Hepatol Commun 2020;4:1392-403.
80. Hagström H, Simon TG, Roelstraete B, Stephansson O, Söderling J, Ludvigsson JF. Maternal obesity increases the risk and severity of NAFLD in offspring. J Hepatol 2021;75:1042-8.
81. Lomas-Soria C, Reyes-Castro LA, Rodríguez-González GL, et al. Maternal obesity has sex-dependent effects on insulin, glucose and lipid metabolism and the liver transcriptome in young adult rat offspring. J Physiol 2018;596:4611-28.
82. Soderborg TK, Clark SE, Mulligan CE, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 2018;9:4462.
83. Lonardo A, Arab JP, Arrese M. Perspectives on precision medicine approaches to NAFLD diagnosis and management. Adv Ther 2021;38:2130-58.
84. Cespiati A, Youngson NA, Tourna A, Valenti L. Genetics and epigenetics in the clinic: precision medicine in the management of fatty liver disease. Curr Pharm Des 2020;26:998-1009.
85. Zelber-Sagi S. Dietary treatment for NAFLD: new clinical and epidemiological evidence and updated recommendations. Semin Liver Dis 2021;41:248-262.
86. Machado MV. What should we advise MAFLD patients to eat and drink? Metab Target Organ Damage 2021;1:9.
87. Semmler G, Datz C, Reiberger T, Trauner M. Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int 2021;41:2249-68.
88. Chang Y, Cho YK, Kim Y, et al. Nonheavy drinking and worsening of noninvasive fibrosis markers in nonalcoholic fatty liver disease: a cohort study. Hepatology 2019;69:64-75.
89. Long MT, Massaro JM, Hoffmann U, Benjamin EJ, Naimi TS. Alcohol use is associated with hepatic steatosis among persons with presumed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2020;18:1831-1841.e5.
90. Åberg F, Puukka P, Salomaa V, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology 2020;71:835-48.
91. Kashiwagi K, Yamaguchi A, Shiba S, et al. Moderate alcohol consumption is not associated with subclinical cardiovascular damage but with hepatic fibrosis in non-alcoholic fatty liver disease. Alcohol 2020;89:1-7.
92. Lange NF, Radu P, Dufour JF. Prevention of NAFLD-associated HCC: role of lifestyle and chemoprevention. J Hepatol 2021;75:1217-27.
93. New-Aaron M, Ganesan M, Dagur RS, Kharbanda KK, Poluektova LY, Osna NA. Pancreatogenic diabetes: triggering effects of alcohol and HIV. Biology (Basel) 2021;10:108.
94. Osna NA, Ganesan M, Seth D, Wyatt TA, Kidambi S, Kharbanda KK. Second hits exacerbate alcohol-related organ damage: an update. Alcohol Alcohol 2021;56:8-16.
95. Osna NA, New-Aaron M, Dagur RS, et al. A review of alcohol-pathogen interactions: new insights into combined disease pathomechanisms. Alcohol Clin Exp Res 2022;46:359-70.
96. Youssef NA, Abdelmalek MF, Binks M, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int 2013;33:1062-70.
97. Gu Y, Zhang W, Hu Y, Chen Y, Shi J. Association between nonalcoholic fatty liver disease and depression: a systematic review and meta-analysis of observational studies. J Affect Disord 2022;301:8-13.
98. Weinstein AA, De Avila L, Kannan S, et al. Interrelationship between physical activity and depression in nonalcoholic fatty liver disease. World J Hepatol 2022;14:612-22.
99. Kleef LA, Hofman A, Voortman T, de Knegt RJ. Objectively measured physical activity is inversely associated with nonalcoholic fatty liver disease: the rotterdam study. Am J Gastroenterol 2022;117:311-8.
100. Chun HS, Lee M, Lee HA, et al. Association of physical activity with risk of liver fibrosis, sarcopenia, and cardiovascular disease in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2022:S1542-3565(22)00001.
101. Adenote A, Dumic I, Madrid C, Barusya C, Nordstrom CW, Rueda Prada L. NAFLD and infection, a nuanced relationship. Can J Gastroenterol Hepatol 2021;2021:5556354.
102. Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol 2011;26:1361-7.
103. Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther 2020;51:216-30.
104. Guaraldi G, Lonardo A, Maia L, Palella FJ Jr. Metabolic concerns in aging HIV-infected persons: from serum lipid phenotype to fatty liver. AIDS 2017;31 Suppl 2:S147-56.
105. Krahn T, Martel M, Sapir-Pichhadze R, et al. Nonalcoholic fatty liver disease and the development of metabolic comorbid conditions in patients with human immunodeficiency virus infection. J Infect Dis 2020;222:787-97.
106. Targher G, Mantovani A, Byrne CD, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut 2020;69:1545-7.
107. Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Invest 2020;50:e13338.
108. Milic J, Barbieri S, Gozzi L, et al. Metabolic-associated fatty liver disease is highly prevalent in the postacute COVID syndrome. Open Forum Infect Dis 2022;9:ofac003.
109. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016;61:1294-303.
110. Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun 2020;4:478-92.
111. Dalekos GN, Gatselis NK, Koukoulis GK. Non-alcoholic steatohepatitis or autoimmune hepatitis? BMJ Case Rep 2020;13:e238400.
112. Dalekos GN, Gatselis NK, Zachou K, Koukoulis GK. NAFLD and autoimmune hepatitis: do not judge a book by its cover. Eur J Intern Med 2020;75:1-9.
113. Kim HJ, Park SJ, Park HK, Byun DW, Suh K, Yoo MH. Association of thyroid autoimmunity with nonalcoholic fatty liver disease in euthyroid middle-aged subjects: a population-based study. J Gastroenterol Hepatol 2022;37:1617-23.
114. Bessone F, Dirchwolf M, Rodil MA, Razori MV, Roma MG. Review article: drug-induced liver injury in the context of nonalcoholic fatty liver disease - a physiopathological and clinical integrated view. Aliment Pharmacol Ther 2018;48:892-913.
115. Allard J, Le Guillou D, Begriche K, Fromenty B. Drug-induced liver injury in obesity and nonalcoholic fatty liver disease. Adv Pharmacol 2019;85:75-107.
116. Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol 2021;18:4-17.
117. Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505-11.
118. Alferink LJM, Radjabzadeh D, Erler NS, et al. Microbiomics, metabolomics, predicted metagenomics, and hepatic steatosis in a population-based study of 1,355 adults. Hepatology 2021;73:968-82.
119. Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054-1062.e5.
120. Yamamura S, Eslam M, Kawaguchi T, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 2020;40:3018-30.
121. Liang Y, Chen H, Liu Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab 2022;107:88-97.
122. Tsutsumi T, Eslam M, Kawaguchi T, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res 2021;51:1115-28.
123. Miao L, Yang L, Guo LS, et al. Metabolic Dysfunction-associated fatty liver disease is associated with greater impairment of lung function than nonalcoholic fatty liver disease. J Clin Transl Hepatol 2022;10:230-7.
124. Lee H, Lee HW, Kim SU, Chang Kim H. Metabolic dysfunction-associated fatty liver disease increases colon cancer risk: a nationwide cohort study. Clin Transl Gastroenterol 2022;13:e00435.
125. Liu Z, Suo C, Shi O, et al. The health impact of MAFLD, a novel disease cluster of NAFLD, is amplified by the integrated effect of fatty liver disease-related genetic variants. Clin Gastroenterol Hepatol 2022;20:e855-75.
126. Quek J, Ng CH, Tang ASP, et al. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality. A Meta-Analysis and Systematic Review of 12 620 736 Individuals. Endocr Pract 2022;28:667-72.
127. Semmler G, Wernly S, Bachmayer S, et al. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)-rather a bystander than a driver of mortality. J Clin Endocrinol Metab 2021;106:2670-7.
128. Nasr P, Blomdahl J, Kechagias S, Ekstedt M. Modifiers of liver-related manifestation in the course of NAFLD. Curr Pharm Des 2020;26:1062-78.
129. Lonardo A. Separating the apples from the oranges: from NAFLD heterogeneity to personalized medicine. Explor Med 2021;2:435-42.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Lonardo A, Singal AK, Osna N, Kharbanda KK. Effect of cofactors on NAFLD/NASH and MAFLD - a paradigm illustrating the pathomechanics of organ dysfunction. Metab Target Organ Damage 2022;2:12. http://dx.doi.org/10.20517/mtod.2022.14
AMA Style
Lonardo A, Singal AK, Osna N, Kharbanda KK. Effect of cofactors on NAFLD/NASH and MAFLD - a paradigm illustrating the pathomechanics of organ dysfunction. Metabolism and Target Organ Damage. 2022; 2(3): 12. http://dx.doi.org/10.20517/mtod.2022.14
Chicago/Turabian Style
Lonardo, Amedeo, Ashwani K. Singal, Natalia Osna, Kusum K. Kharbanda. 2022. "Effect of cofactors on NAFLD/NASH and MAFLD - a paradigm illustrating the pathomechanics of organ dysfunction" Metabolism and Target Organ Damage. 2, no.3: 12. http://dx.doi.org/10.20517/mtod.2022.14
ACS Style
Lonardo, A.; Singal AK.; Osna N.; Kharbanda KK. Effect of cofactors on NAFLD/NASH and MAFLD - a paradigm illustrating the pathomechanics of organ dysfunction. Metab Target Organ Damage. 2022, 2, 12. http://dx.doi.org/10.20517/mtod.2022.14
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 57 clicks
Cite This Article 57 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.