Screening for advanced liver fibrosis in overweight and obese patients with NAFLD
Globally, cirrhosis is the leading cause of liver-related mortality[1]. Deaths due to cirrhosis accounted for 2.4% of total deaths globally in 2017 compared with 1.9% in 1990. Furthermore, cirrhosis caused by non-alcoholic steatohepatitis (NASH) steadily increased, while most other causes of cirrhosis decreased[2]. Thus, soon NASH may overtake viral hepatitis as the main cause of cirrhosis. As NASH is difficult to diagnose, requires liver biopsy in most cases, and develops from non-alcoholic fatty liver (NAFL), the focus on non-alcoholic fatty liver disease (NAFLD), including NAFL and NASH[3], is of major clinical and scientific interest in the pathogenesis of cirrhosis. However, the natural history of NAFLD is heterogeneous. Several main mechanisms are considered to be involved in its pathogenesis, including liver-related genetic risk, increased hepatic de-novo lipogenesis, gut dysbiosis and inflammation and increase of adipose tissue in the visceral compartment which is associated with increased release of fatty acids and cytokines and dysregulated release of adipokines[4-10].
NAFLD is an important risk factor for hepatocellular carcinoma[11], type 2 diabetes[12] and cardiovascular disease[13] and represents an important cause and complication of liver transplantation[14]. Although patients with NAFL can develop NASH and progressive fibrosis, which puts them at an increased risk of morbidity and mortality, only fibrosis, but no other histological liver characteristics, was shown to independently predict increased all-cause and disease-specific mortality in patients with NAFLD[15-17]. Furthermore, among the different stages of fibrosis, fibrosis stages F3 and F4 were particularly associated with increased risks of liver-related complications and death[18].
Liver biopsy is the gold standard for the assessment of liver fibrosis[19]. However, it has several limitations such as sampling error and complications due to its invasive nature[20]. Therefore, there is a large interest in identifying noninvasive methods and tests to estimate liver fibrosis. Among them are blood-based markers, clinical scores and imaging-based markers of liver fibrosis[21,22]. While most of the blood-based markers and clinical scores of fibrosis are widely available and, in most cases, relatively cheap, the imaging-based markers of fibrosis are quite expensive. Kanwal and colleagues’ call to action[23], and the associated clinical care pathway[24], suggested a global guiding strategy to promote the early diagnosis of NAFLD and NASH, starting in the primary care clinic. Three high-risk groups of people were identified by the task force: people with diabetes, those with metabolic syndrome, and people with steatosis or increased concentrations of plasma aminotransferases (ALT and/or AST), or both[24]. Furthermore, the Fibrosis-4 (FIB-4) index was selected as the initial screening tool. This pathway is intended to be used in settings where care for patients with NAFLD is provided, including primary care, endocrine, obesity medicine, and gastroenterology practices.
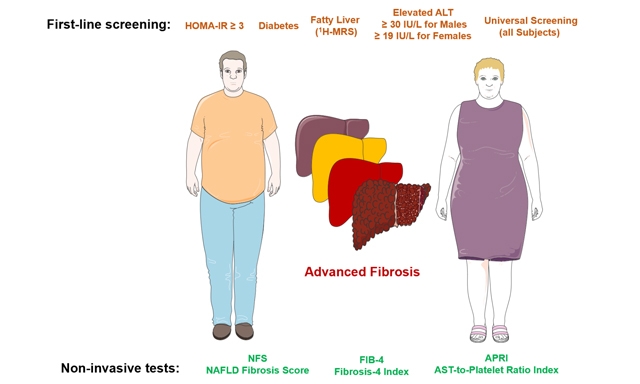
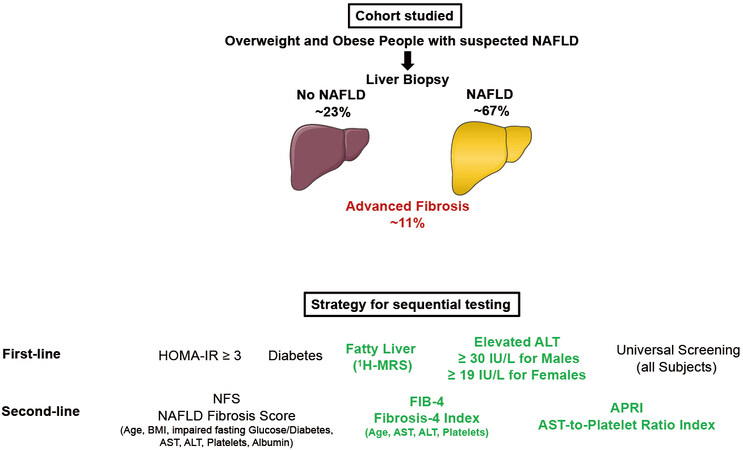
Following up on these recommendations derived from experts’ opinions, Bril, Cusi and colleagues now undertook an important study evaluating the performance of different strategies to select patients at high risk of advanced liver fibrosis (F3 and F4) among overweight and obese subjects[25]. For this purpose, they analyzed data from a total of 275 overweight and obese patients who were recruited from hepatology and endocrinology clinics at the University of Florida in Gainesville, FL and the University of Texas Health Science Center at San Antonio (UTHSCSA) in San Antonio, TX, as well as from the general population. When NAFLD was diagnosed, patients had a percutaneous liver biopsy. The authors identified 29 patients with advanced fibrosis. Five selection strategies were compared to determine the best screening algorithm: (1) a “metabolic approach”: selecting patients based on HOMA-IR ≥ 3; (2) a “diabetes approach”: selecting only patients with type 2 diabetes; (3) an “imaging approach”: selecting patients with hepatic steatosis based on 1H-magnetic resonance spectroscopy (MRS); (4) a “liver biochemistry approach”: selecting patients with elevated ALT (i.e., ≥ 30 IU/L for males and ≥ 19 IU/L for females); and (5) universal screening of all overweight and obese patients. FIB-4 index, NAFLD fibrosis score (NFS), and APRI (AST-to-platelet ratio index) were applied as screening strategies. Three important findings were derived from this study. First, among the noninvasive tests in a universal screening approach, the best performance had APRI, with 24 patients from 100 requiring a liver biopsy and a number of biopsies per patient identified with advanced fibrosis of 3.05. Second, universal screening in overweight and obese subjects, even with the APRI, is not justified as it would result in a higher number of false positive results compared to more restrictive strategies. Among the best strategic approaches in overweight and obese subjects were the application of APRI in patients with elevated ALT levels (24 patients from 100 requiring a liver biopsy and a number of biopsies per patient identified with advanced fibrosis of 2.95) and in patients with NAFLD diagnosed by
With their present work, Bril, Cusi and colleagues follow up on their important studies[26-28] showing that the prevalence of 20% of moderate-to-advanced fibrosis in patients with type 2 diabetes is twice as high as in patients with steatosis but without diabetes. Furthermore, they found that one in six patients with type 2 diabetes and unknown NAFLD had moderate-to-advanced fibrosis. In addition, they showed that imaging, e.g., transient elastography, and diagnostic panels, e.g., FIB-4 index and APRI, are very effective in identifying moderate-to-advanced fibrosis in this high-risk population[26].
A few caveats should be highlighted. The authors did not intend to suggest a screening strategy for the “real world” but rather assess the performance in risk groups and testing against liver histology in patients recruited at a tertiary university hospital research setting, as this was not a population-based screening study. Many had elevated plasma ALT and the cutoffs chosen (i.e., ≥ 30 IU/L for males and ≥ 19 IU/L for females) were lower than those in clinical practice (e.g., ≥ 40 U/L), enhancing the sensitivity and overall performance of the liver biochemistry approach. However, most patients in primary care settings have ALT < 40 U/L. Because plasma ALT > 30 U/L is associated with increased liver morbidity and mortality, as a practical approach for clinicians, the clinical practice guidelines have recently chosen as a practical approach for clinicians a lower ALT (> 30 U/L) for both genders as a high-risk group for NAFLD and advanced fibrosis[29]. While APRI > 0.50 performed well overall and was comparable to FIB-4 > 1.3, it should be noted that the specificity and overall performance of FIB-4 can be improved for FIB-4 using higher cutoffs (e.g., 1.67)[28]. Liver assessment is nowadays widely done in the clinic by transient elastography and measurement of liver content fat by MRI-based techniques is not recommended (also done by the investigators as part of research studies). Finally, HOMA-IR was also part of the research setting of the investigators but should not be at present part of a routine NAFLD screening strategy as there is significant variability among insulin assays by clinical laboratories, which will diminish its performance in the real world.
In conclusion, for overweight and obese patients with metabolic syndrome and suspected NAFLD, screening for advanced hepatic fibrosis is warranted using noninvasive tests for this purpose, e.g., FIB-4 or APRI. Targeting screening of patients with elevated ALT levels using FIB-4 or APRI provides the most cost-effective first-line approach [Figure 1]. Still, because in most patients plasma aminotransferases are not elevated[26-28], current guidelines[24,29] recommend FIB-4 (over APRI or NFS) to identify advanced fibrosis in high-risk patients given the superior screening and long-term outcomes predictive value of FIB-4[29-31]. Future research is warranted to better stratify subjects with suspected NAFLD regarding duration of diabetes, quality of blood glucose control and body fat distribution.
Figure 1. Cohort studied and methods used for sequential screening for advanced fibrosis. NAFLD: Non-alcoholic fatty liver disease;
DECLARATIONS
Author’s contributionThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author 2022.
REFERENCES
1. 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88.
2. 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245-66.
4. Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 2012;142:711-725.e6.
5. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908-22.
6. Romeo S, Sanyal A, Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metab 2020;31:35-45.
7. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol 2020;8:616-27.
8. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol 2021;6:578-88.
9. Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol 2022;10:284-96.
10. Lonardo A, Singal AK, Osna N, Kharbanda KK. Effect of cofactors on NAFLD/NASH and MAFLD. A paradigm illustrating the pathomechanics of organ dysfunction. Metab Target Organ Damage 2022;2:12.
11. Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969-977.e2.
12. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 2021;70:962-9.
13. Mantovani A, Csermely A, Petracca G, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021;6:903-13.
14. Lonardo A, Mantovani A, Petta S, Carraro A, Byrne CD, Targher G. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation. Nat Rev Endocrinol 2022;18:638-50.
15. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-54.
16. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389-97.e10.
17. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557-65.
18. Sanyal AJ, Van Natta ML, Clark J, et al. NASH Clinical Research Network (CRN). Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559-69.
20. Davison BA, Harrison SA, Cotter G et al. , Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 2020;73:1322-32.
21. Tamaki N, Kurosaki M, Huang DQ, Loomba R. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol Res 2022;52:497-507.
22. Kim BK, Tamaki N, Imajo K, et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J Hepatol 2022;77:1482-90.
23. Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH epidemic: a call to action. Metabolism 2021;122:154822.
24. Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657-69.
25. Bril F, Godinez Leiva E, Lomonaco R, et al. Assessing strategies to target screening for advanced liver fibrosis among overweight and obese patients. Metab Target Organ Damage 2022;2:11.
26. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399-406.
27. Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity 2021;29:1950-60.
28. Bril F, McPhaul MJ, Caulfield MP, et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care 2020;43:290-7.
29. Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American association for the study of liver diseases (AASLD). Endocr Pract 2022;28:528-62.
30. Younes R, Caviglia GP, Govaere O, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol 2021;75:786-94.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Stefan N. Screening for advanced liver fibrosis in overweight and obese patients with NAFLD. Metab Target Organ Damage 2022;2:21. http://dx.doi.org/10.20517/mtod.2022.29
AMA Style
Stefan N. Screening for advanced liver fibrosis in overweight and obese patients with NAFLD. Metabolism and Target Organ Damage. 2022; 2(4): 21. http://dx.doi.org/10.20517/mtod.2022.29
Chicago/Turabian Style
Stefan, Norbert. 2022. "Screening for advanced liver fibrosis in overweight and obese patients with NAFLD" Metabolism and Target Organ Damage. 2, no.4: 21. http://dx.doi.org/10.20517/mtod.2022.29
ACS Style
Stefan, N. Screening for advanced liver fibrosis in overweight and obese patients with NAFLD. Metab Target Organ Damage. 2022, 2, 21. http://dx.doi.org/10.20517/mtod.2022.29
About This Article
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.